Patents
Literature
32 results about "Treatment-resistant depression" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Treatment-resistant depression (TRD) is a term used in clinical psychiatry to describe a condition that affects people with major depressive disorder (MDD) who do not respond adequately to a course of appropriate antidepressant medication within a certain time. Typical definitions of TRD vary, and they do not include a resistance to psychological therapies. Inadequate response has traditionally been defined as no clinical response whatsoever (e.g. no improvement in depressive symptoms). However, many clinicians consider a response inadequate if the person does not achieve full remission of symptoms. People with treatment-resistant depression who do not adequately respond to antidepressant treatment are sometimes referred to as pseudoresistant. Some factors that contribute to inadequate treatment are: early discontinuation of treatment, insufficient dosage of medication, patient noncompliance, misdiagnosis, and concurrent psychiatric disorders. Cases of treatment-resistant depression may also be referred to by which medications people with TRD are resistant to (e.g.: SSRI-resistant). In TRD adding further treatments such as psychotherapy, lithium, or aripiprazole is weakly supported as of 2019.
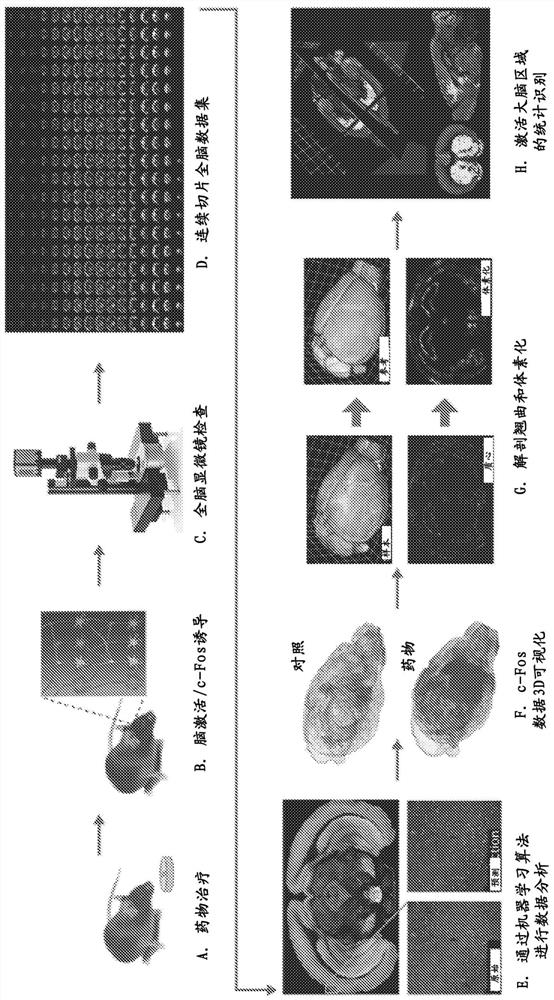
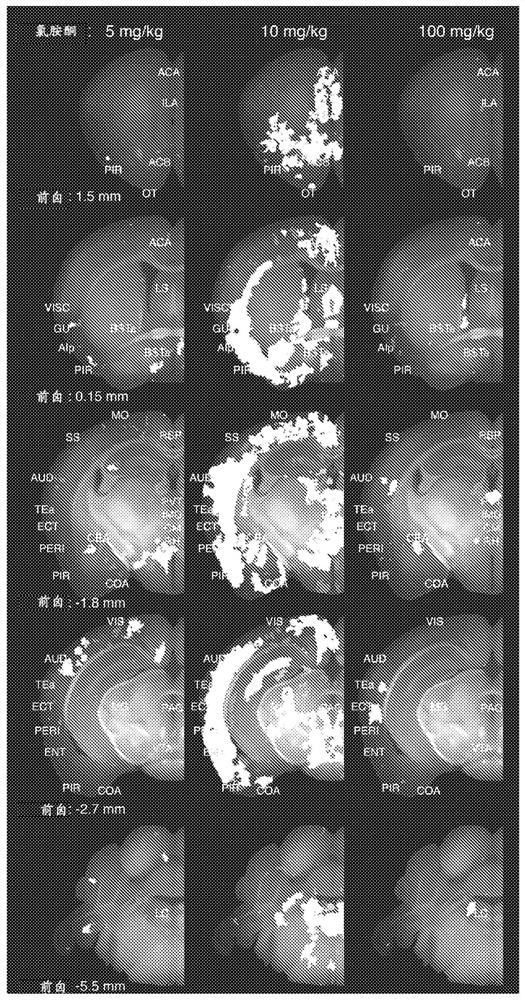
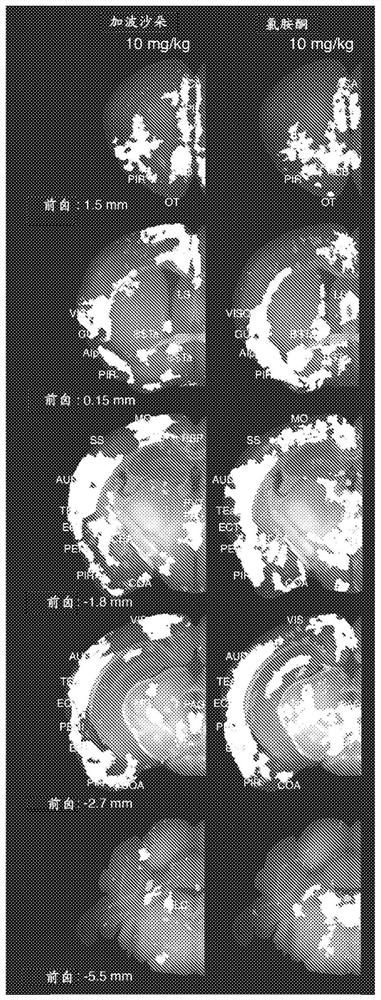
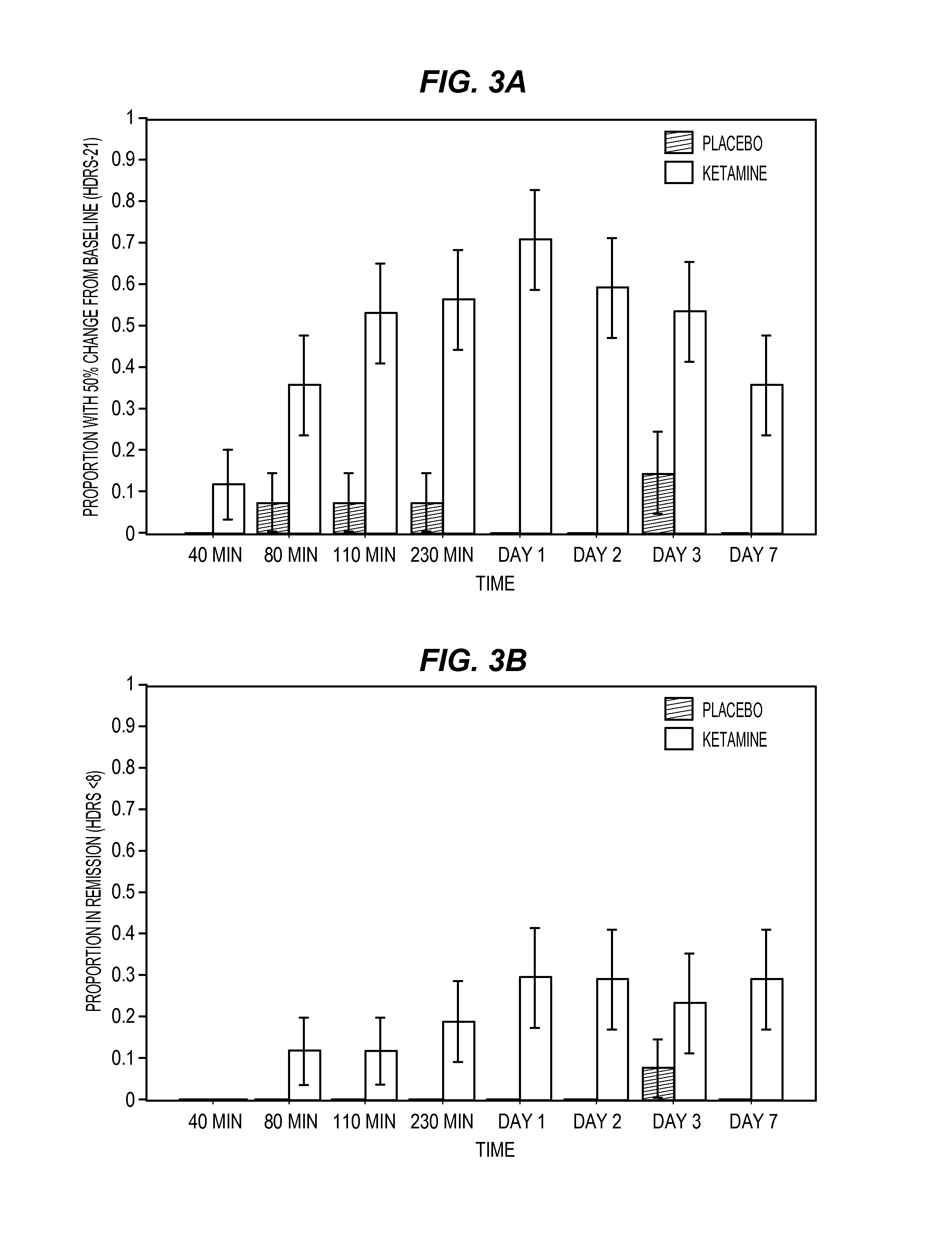
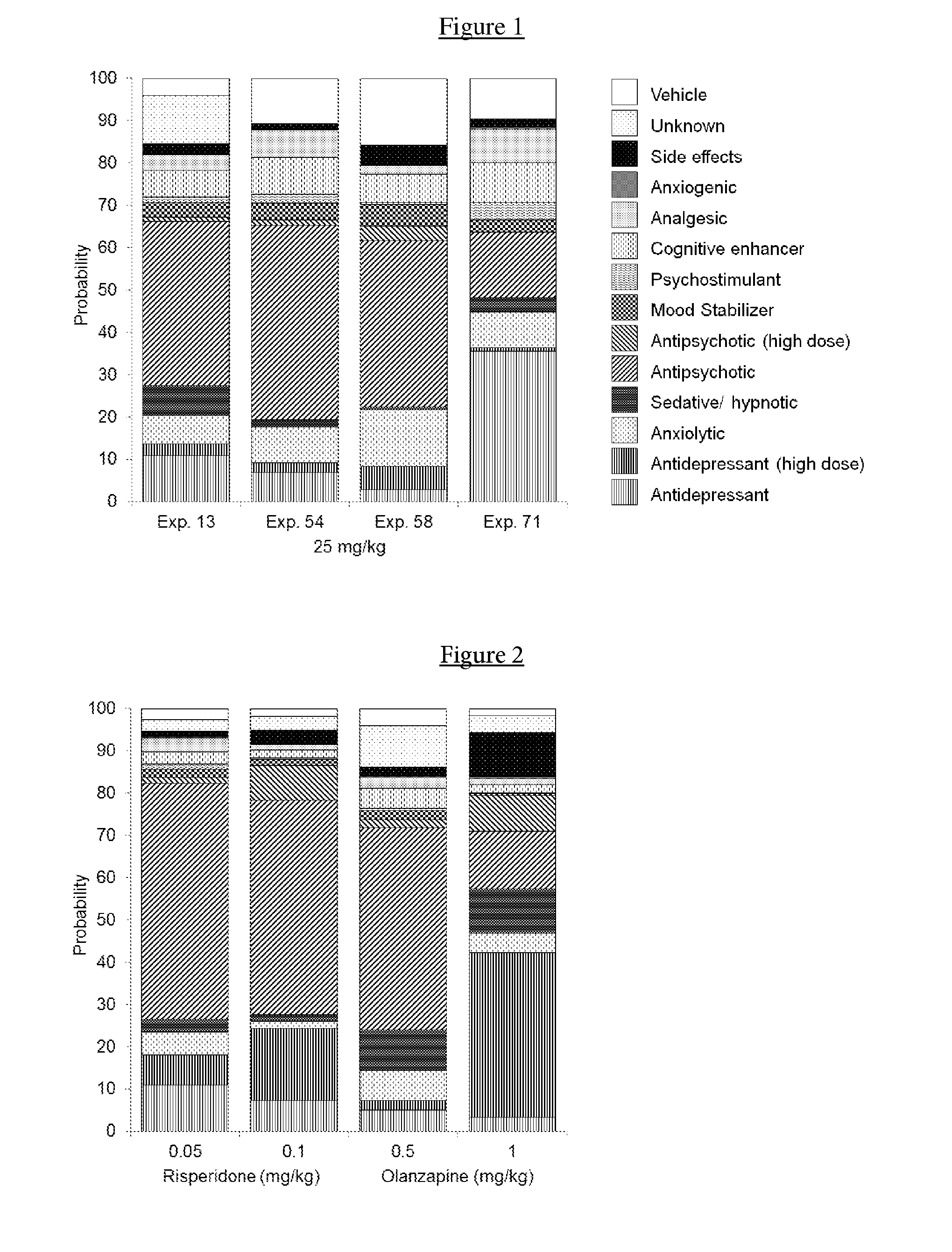
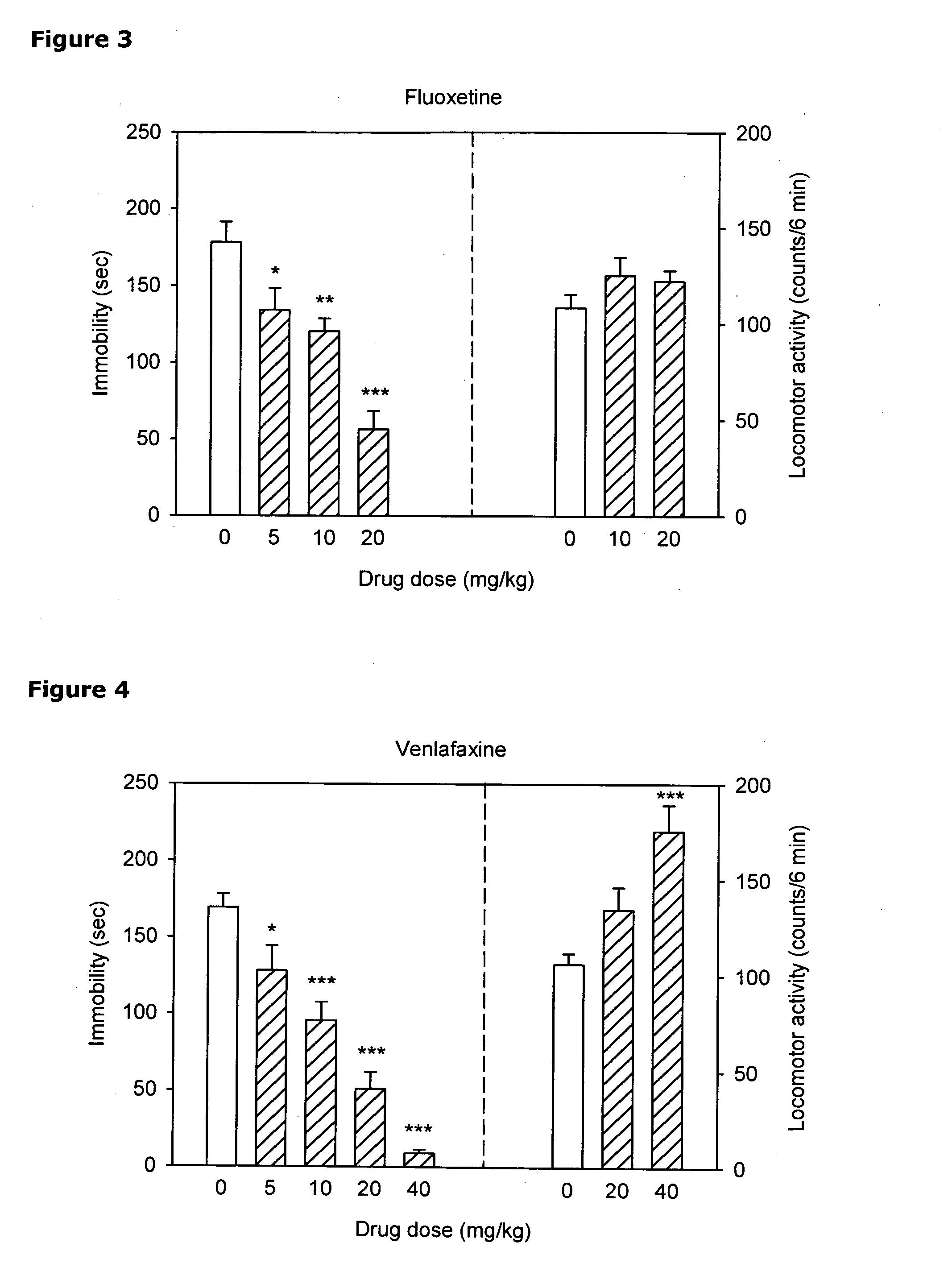
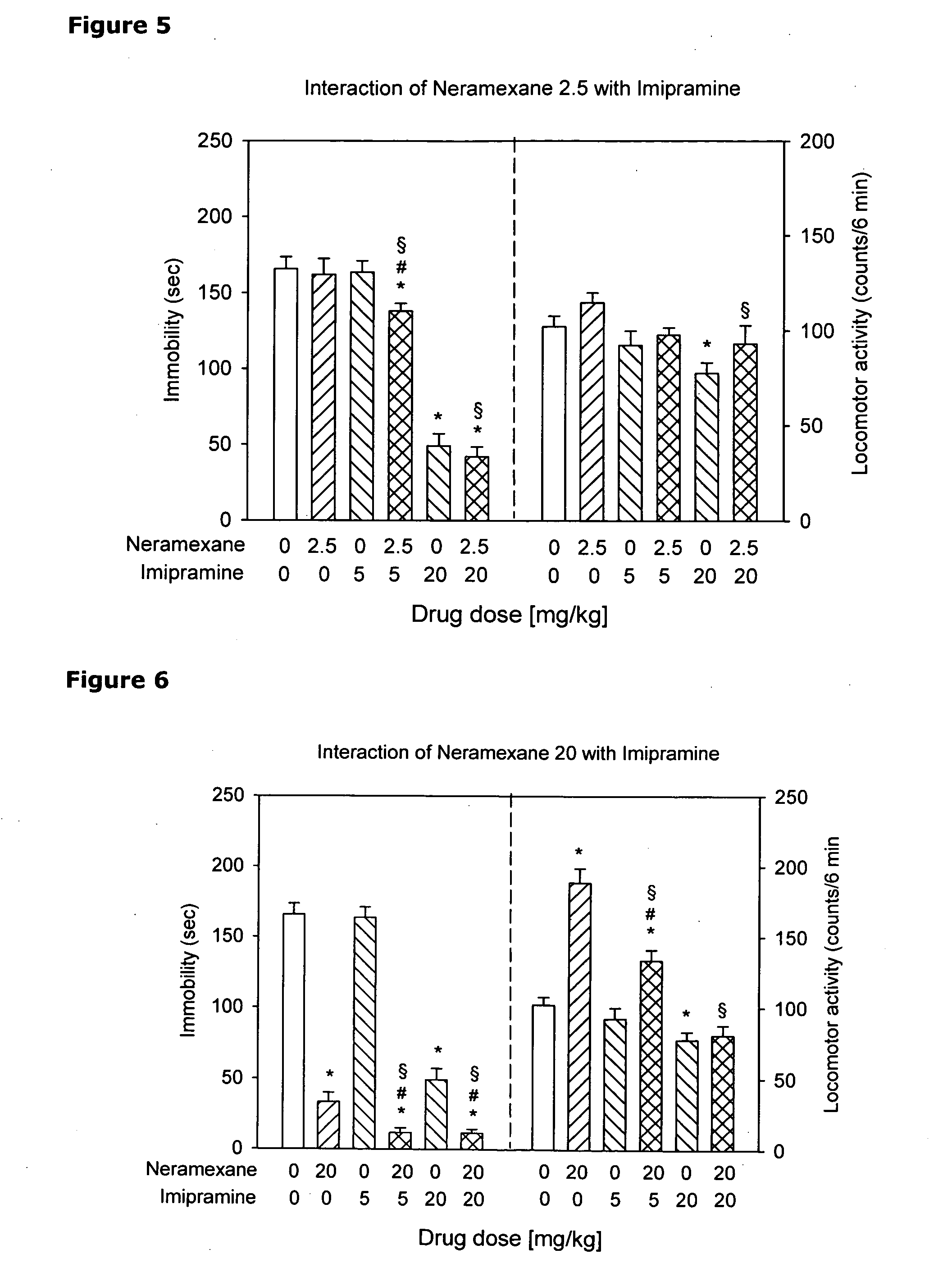
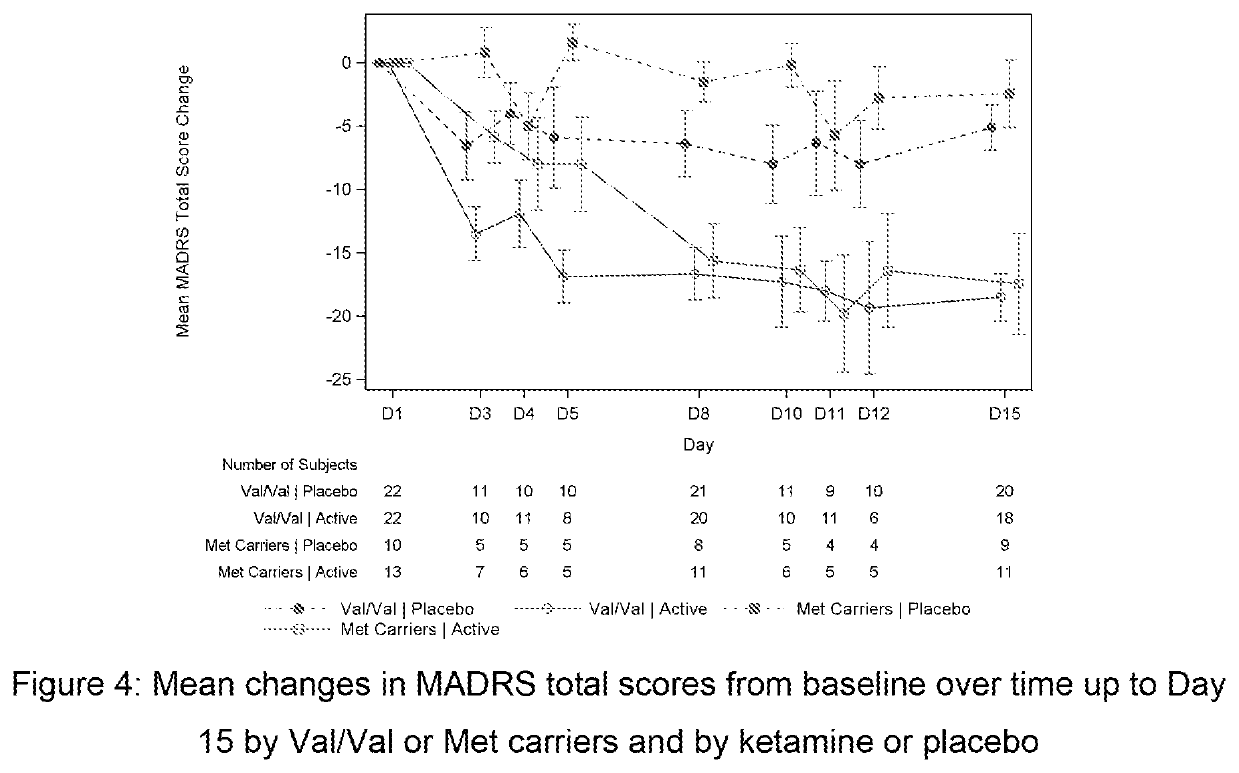
Intranasal administration of ketamine to treat depression
ActiveUS8785500B2Minor adverse side effectAct quicklyBiocideOrganic active ingredientsEnteral administrationKetamine
Methods and compositions for the treatment of treatment-resistant depression are described. More specifically, the invention demonstrates that intranasal administration of ketamine is effective to ameliorate the symptoms of depression in a patient who has not responded to an adequate trial of one antidepressant in the current episode and has recurrent or chronic depressive symptoms (>2 years).
Owner:U S GOVERNMENT REPRESENTED BY THE DEPT OF VETERANS AFFAIRS +3
Combination of an NMDA receptor antagonist and a selective serotonin reuptake inhibitor for the treatment of depression and other mood disorders
The present invention provides a method for the treatment of depression, including treatment-resistant depression, and other mood disorders using a combination of an NMDA receptor antagonist and a SSRI that is citalopram or escitalopram. It has unexpectedly been shown that the combination has a synergistic and potentiated effect of either compound as monotherapy, resulting in an enhanced therapeutic effect at lower doses.
Owner:FOREST LAB HLDG LTD +1
Assays For Selecting A Treatment Regimen For A Subject With Depression And Methods For Treatment
InactiveCN104053785ANervous disorderMicrobiological testing/measurementTest sampleSerotonin receptor inhibitors
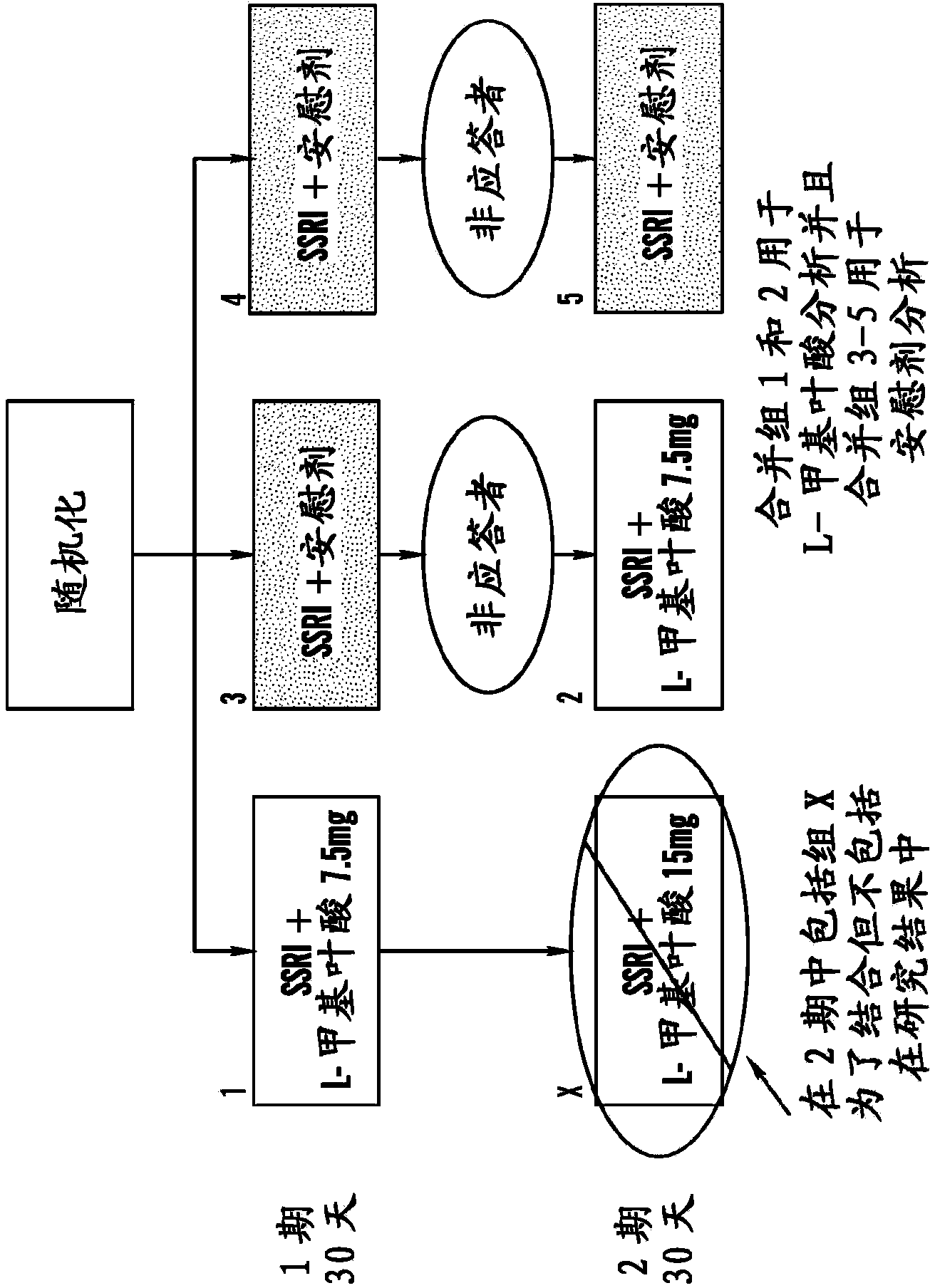
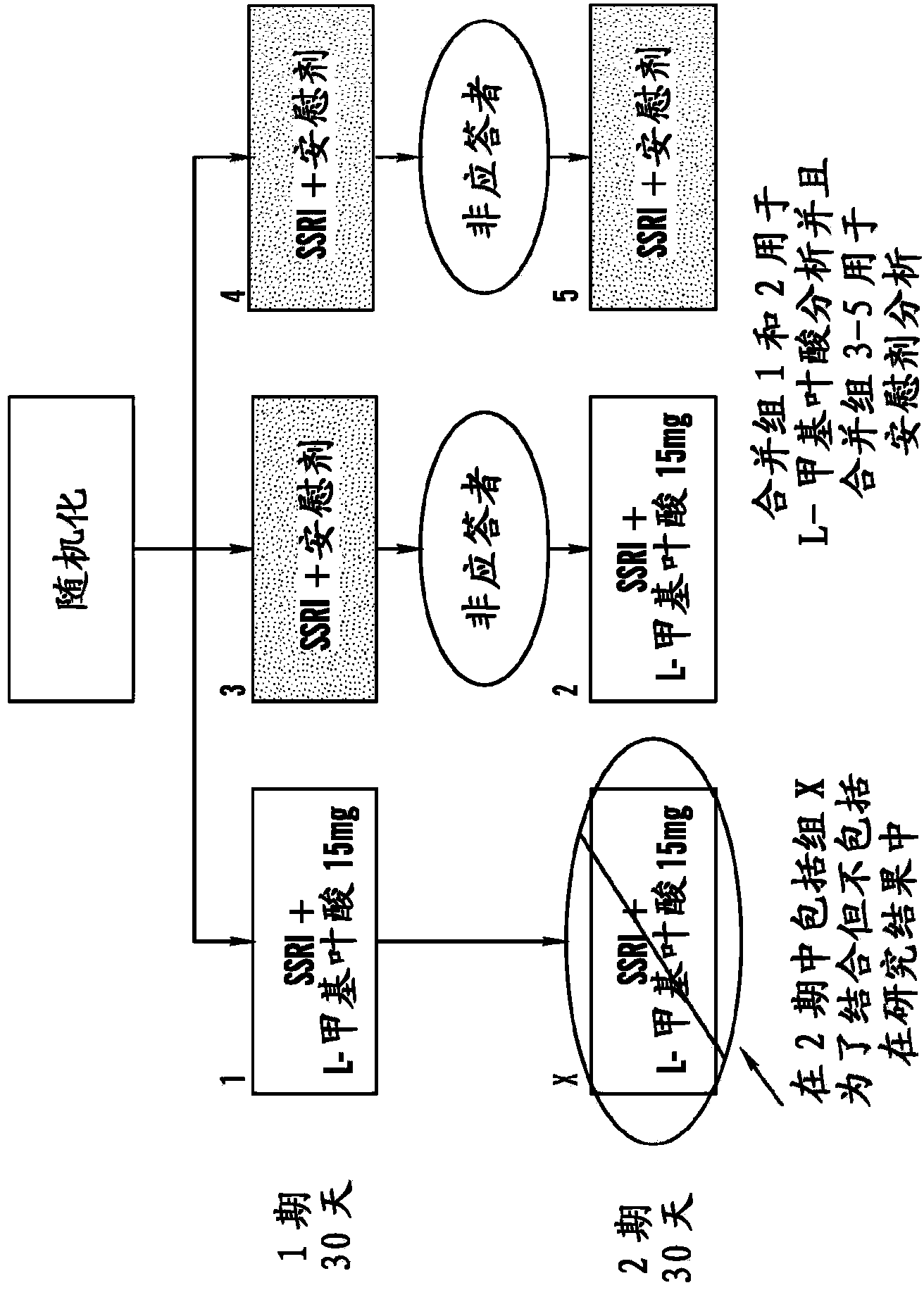
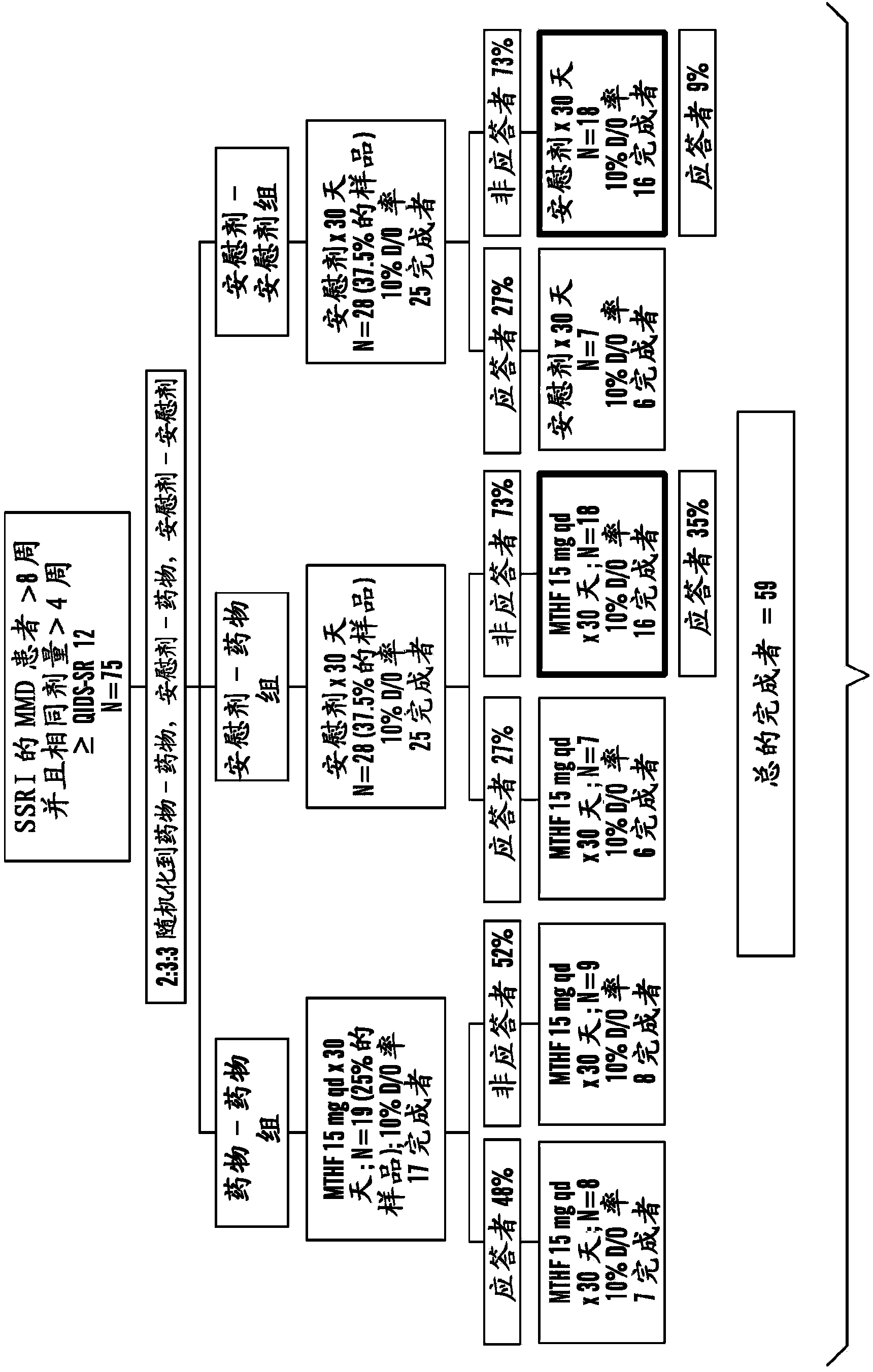
Disclosed herein are novel assays, systems and kits for selecting a treatment regimen for a subject with depression by identifying at least one nucleic acid polymorphism, e.g., but not limited to, at the MTHFR, MTR, or MTRR locus, and / or determining expression levels of peripheral biomarkers (e.g., SAM, SAH, and 4-HNE) in a test sample from a human subject. These biomarkers can be used to determine the effectiveness of treating a depressed subject with a folate-containing compound (alone or as an adjunct to an antidepressant). Additionally, these biomarkers can be used to select an appropriate treatment regimen for subjects with treatment-resistant depression (e.g., resistant to at least one selective serotonin reuptake inhibitor). Methods and compositions for treating a subject with depression and / or determining or improving the effectiveness of an antidepressant drug taken by a subject are also provided herein.
Owner:ALFASIGMA SPA
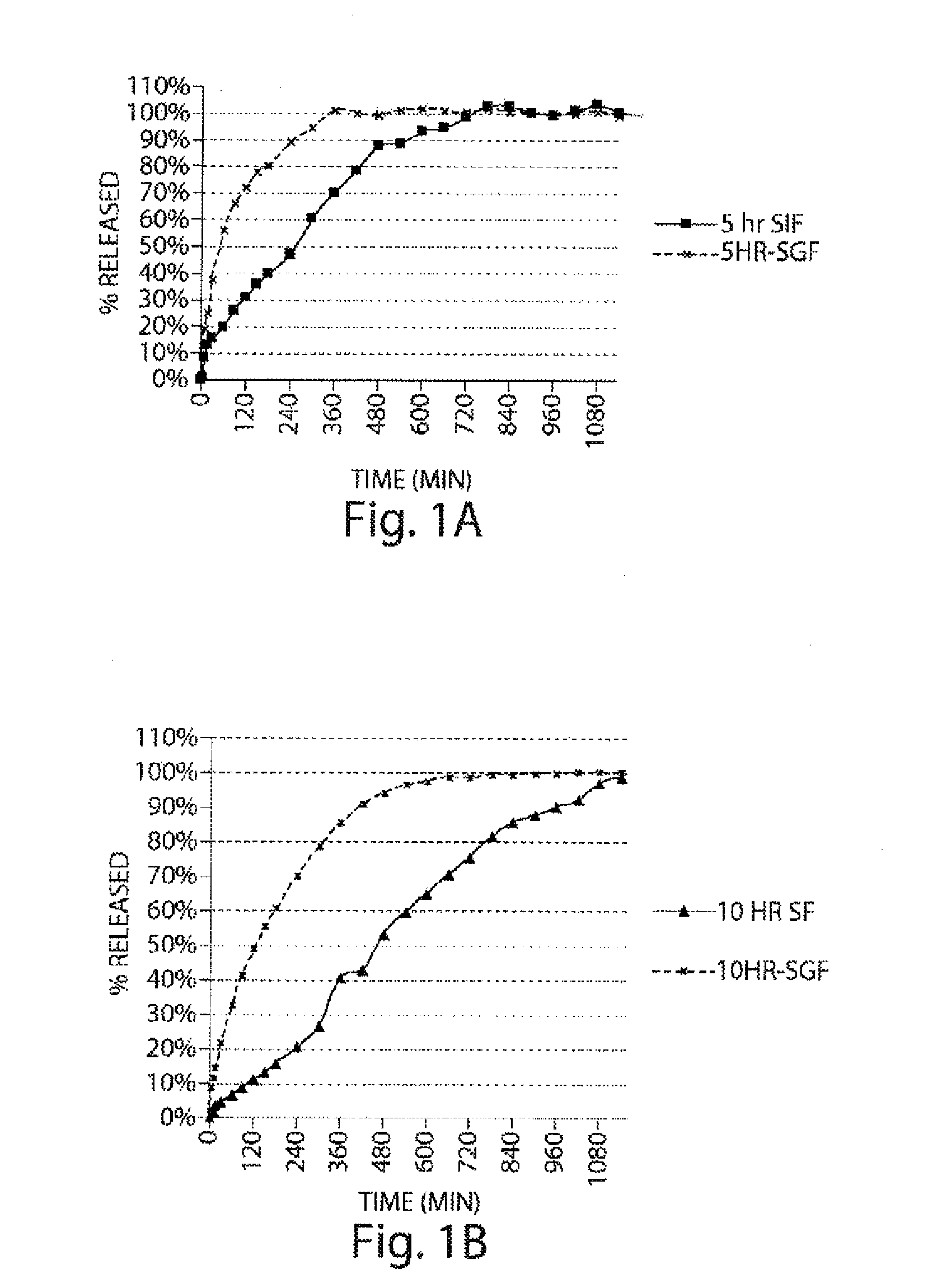
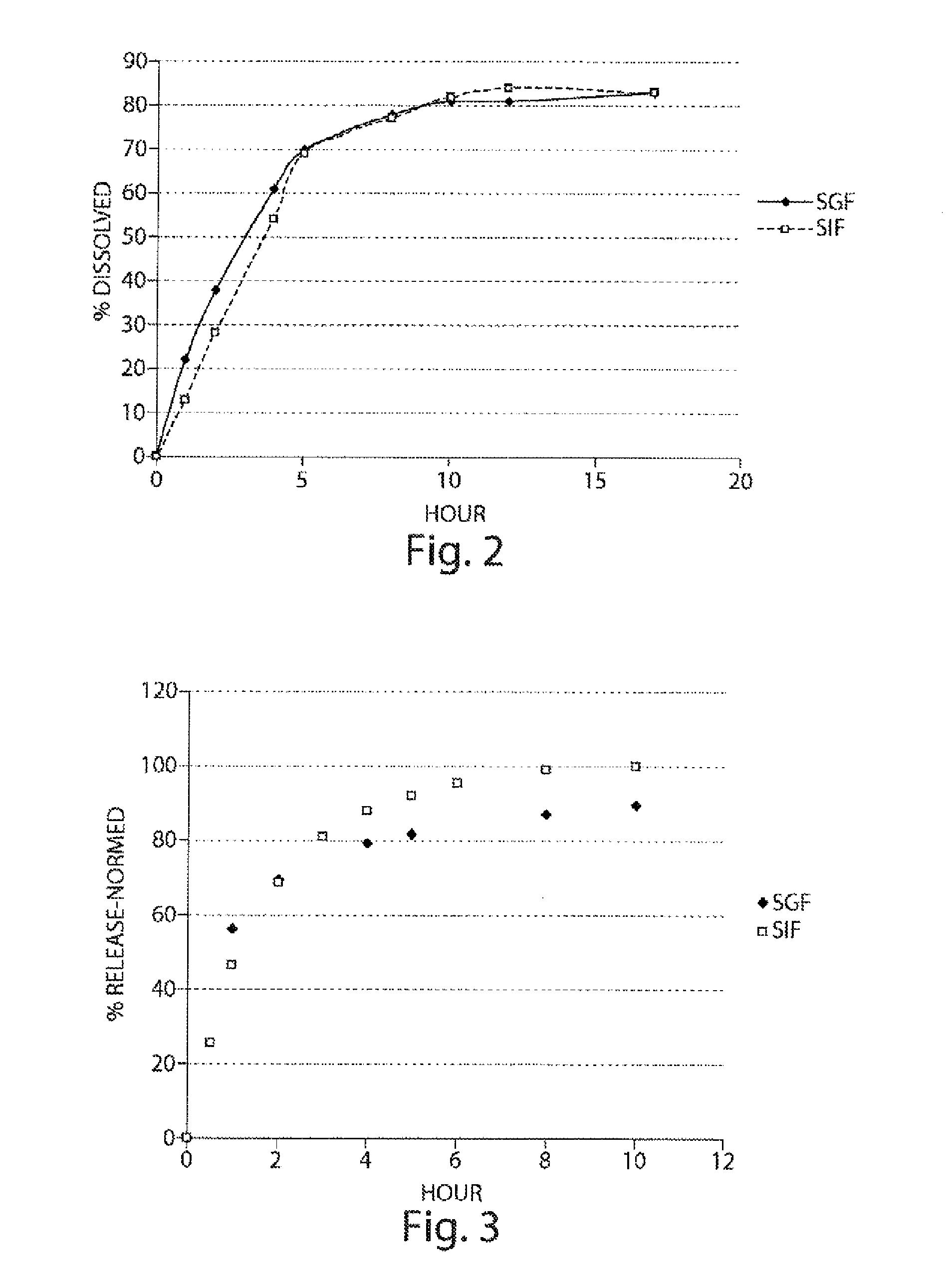
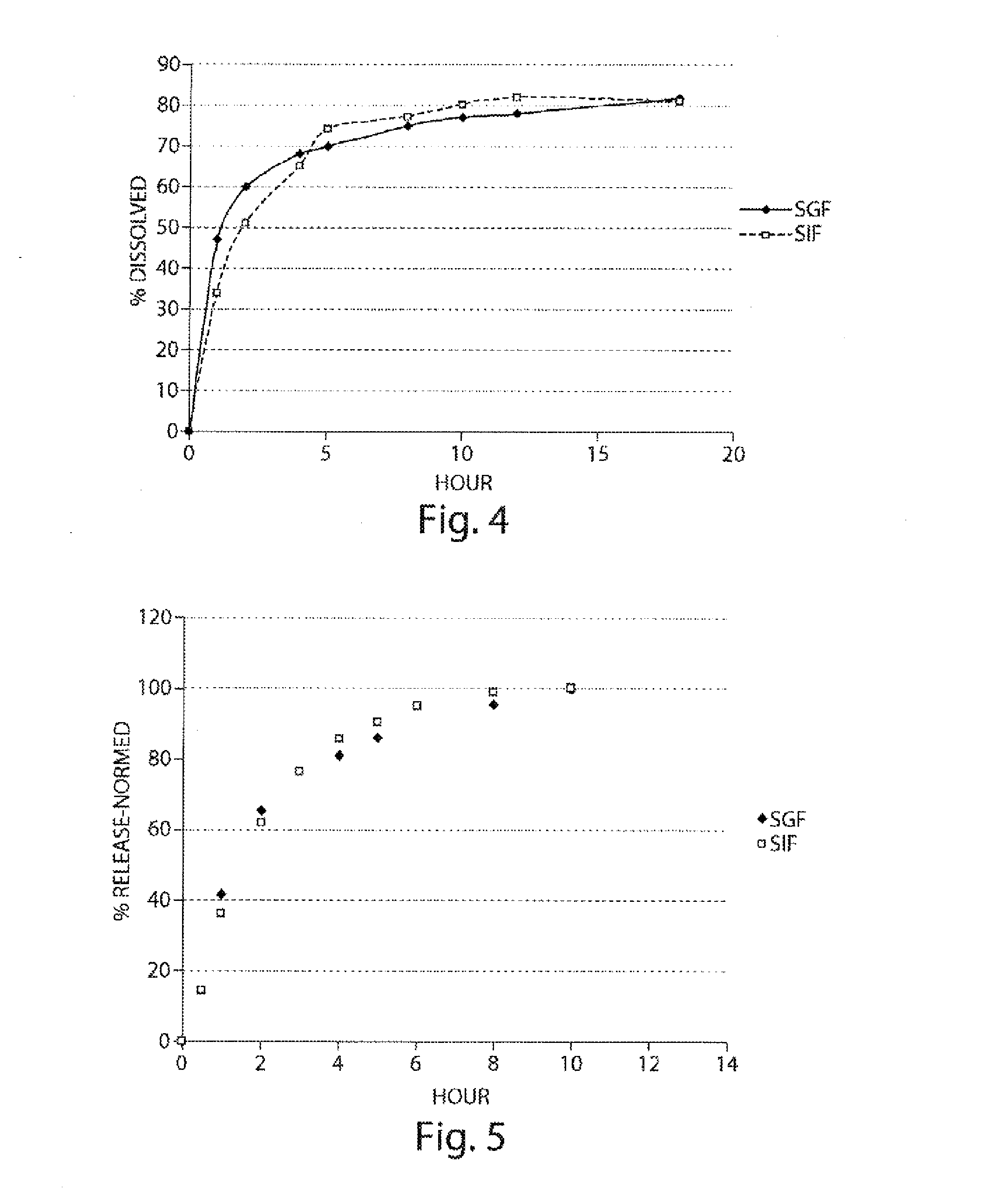
Pharmaceutical compositions of metabotropic glutamate 5 receptor (MGLU5) antagonists
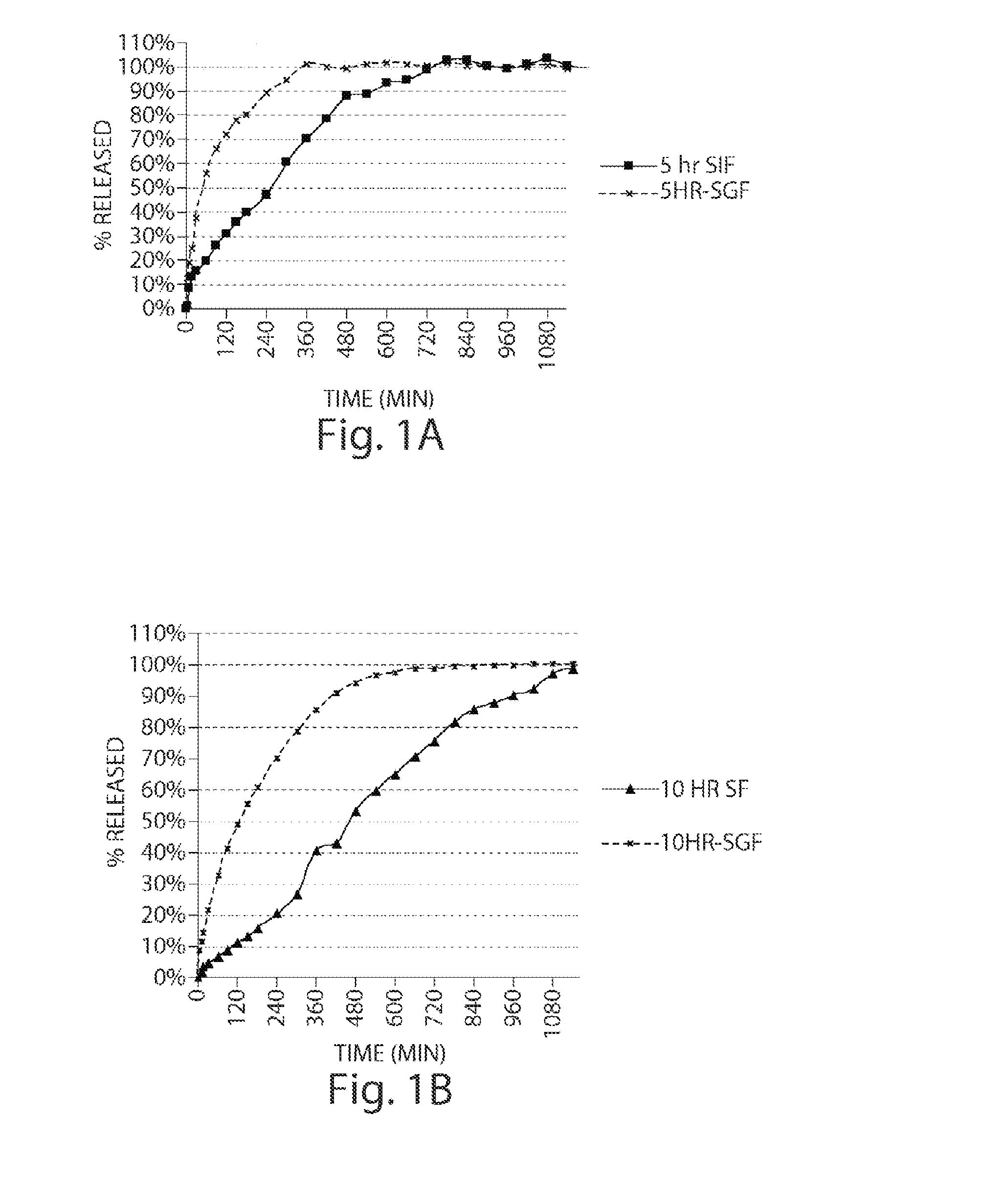
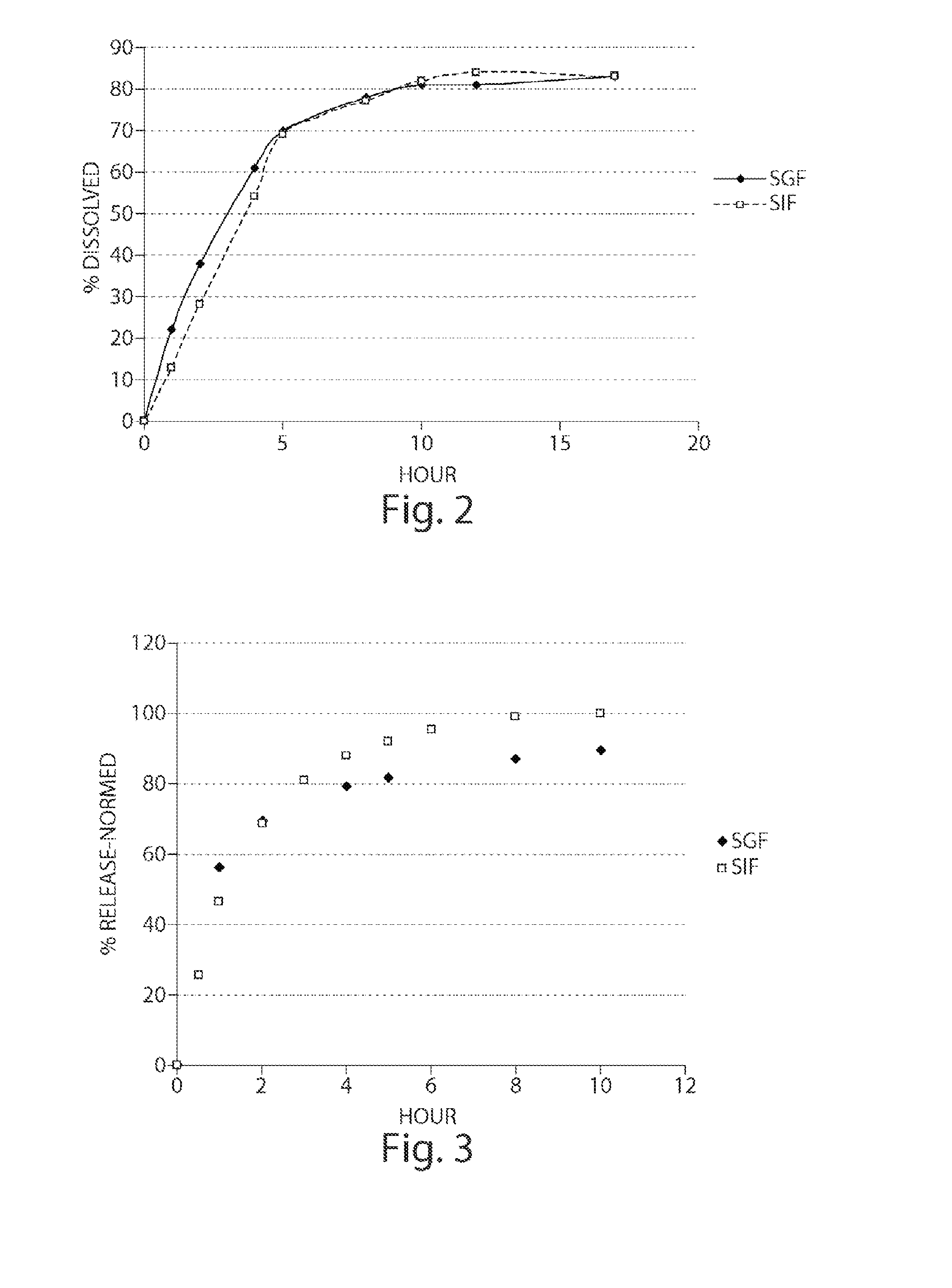
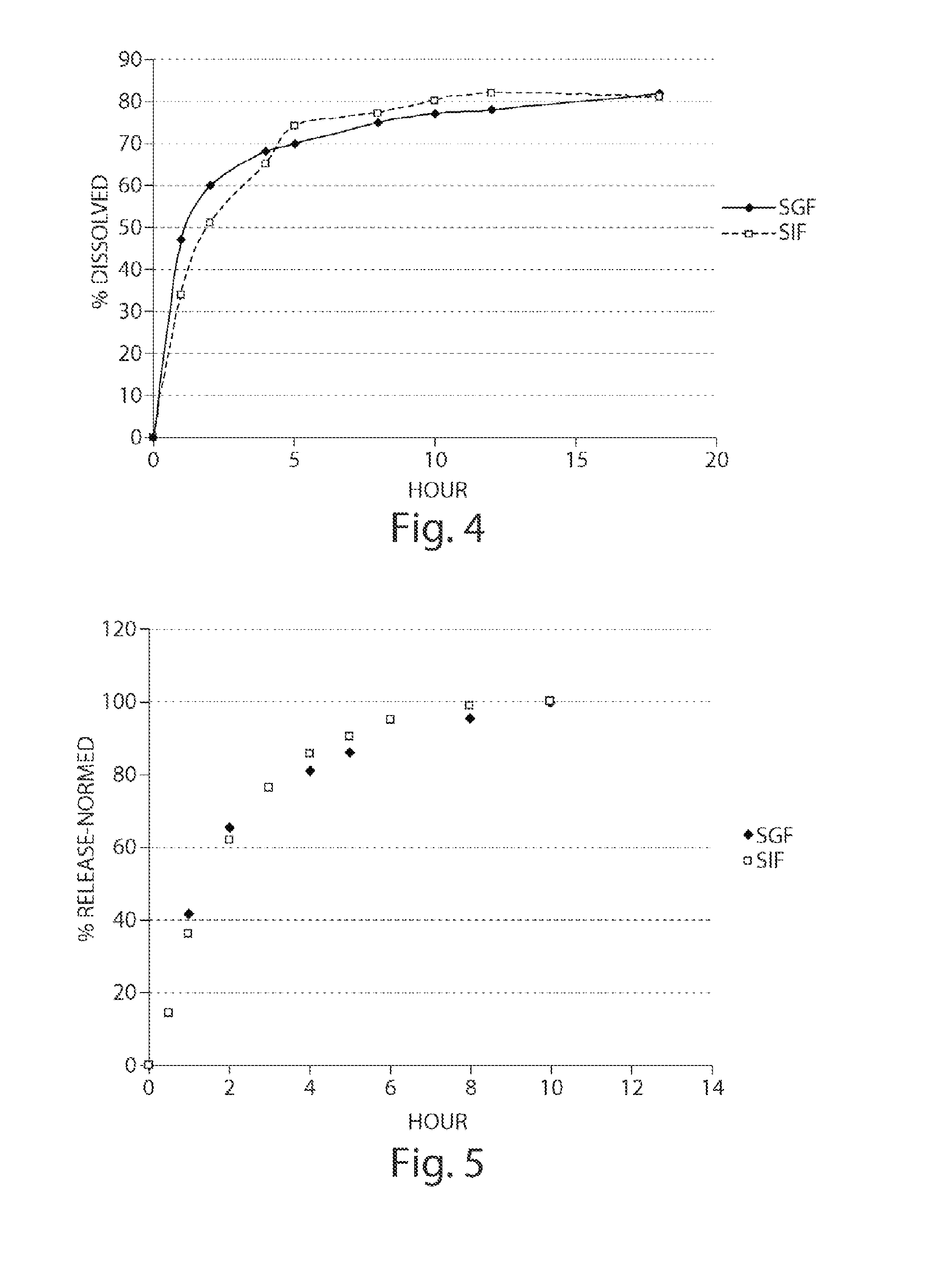
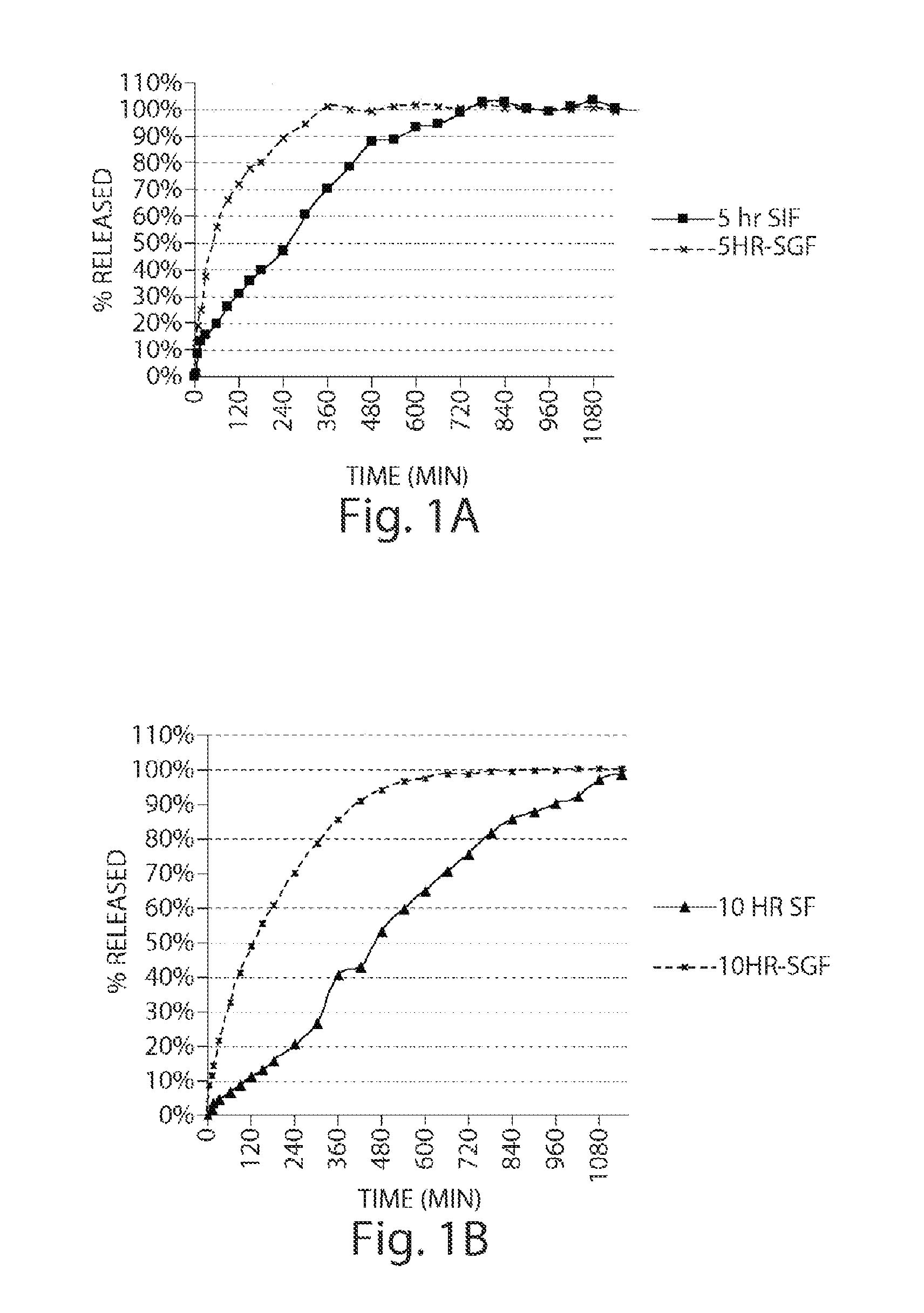
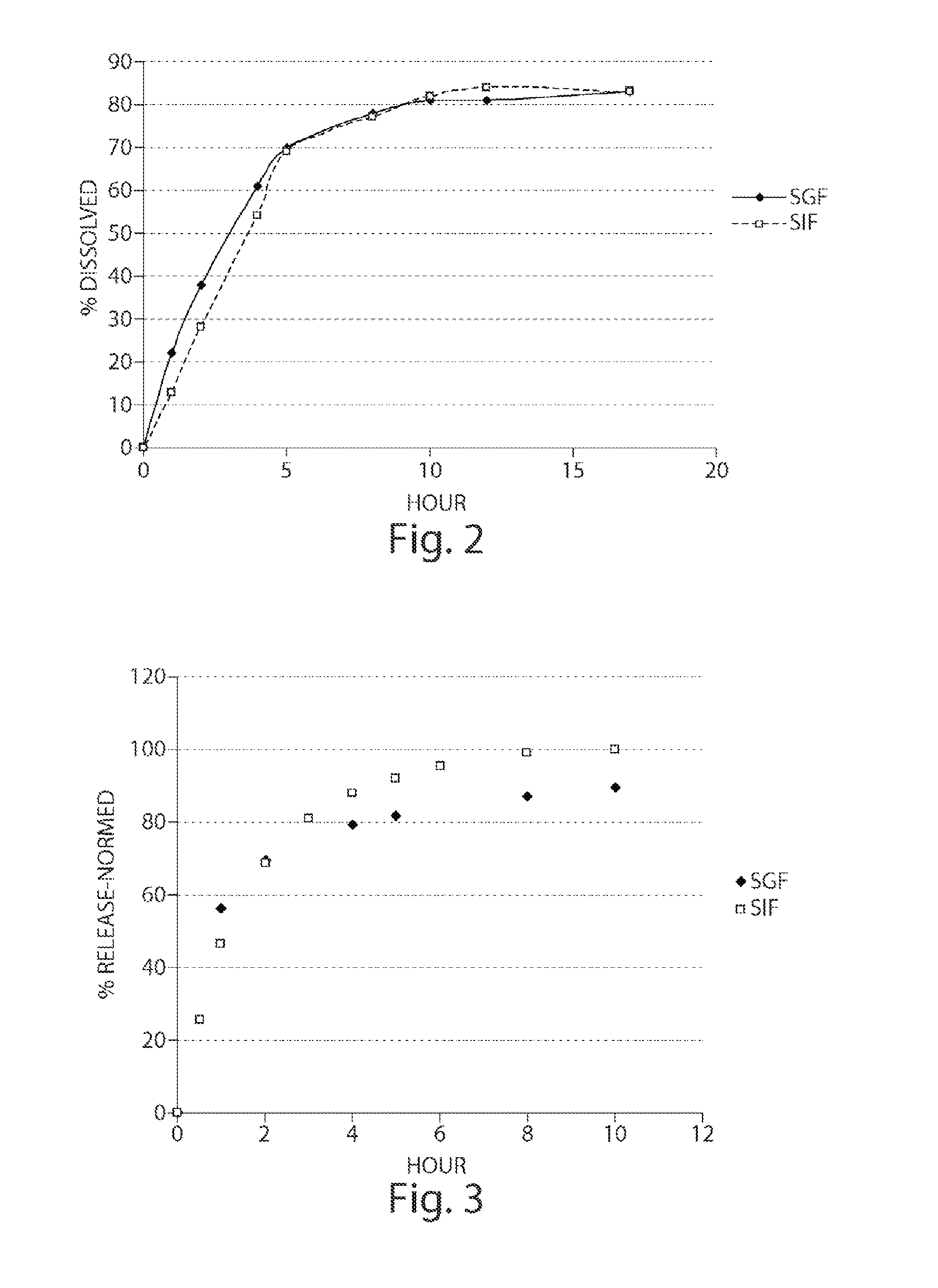
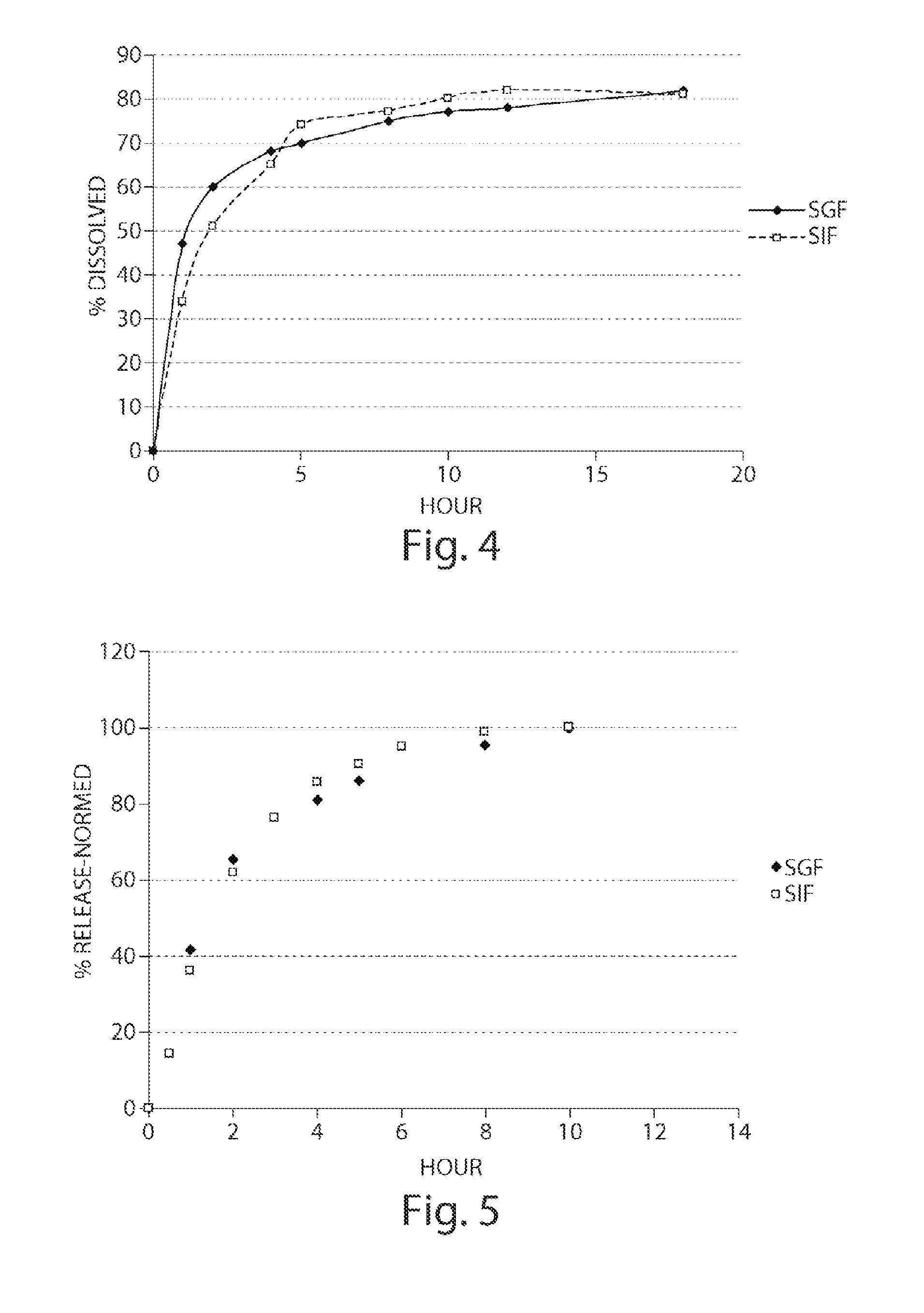
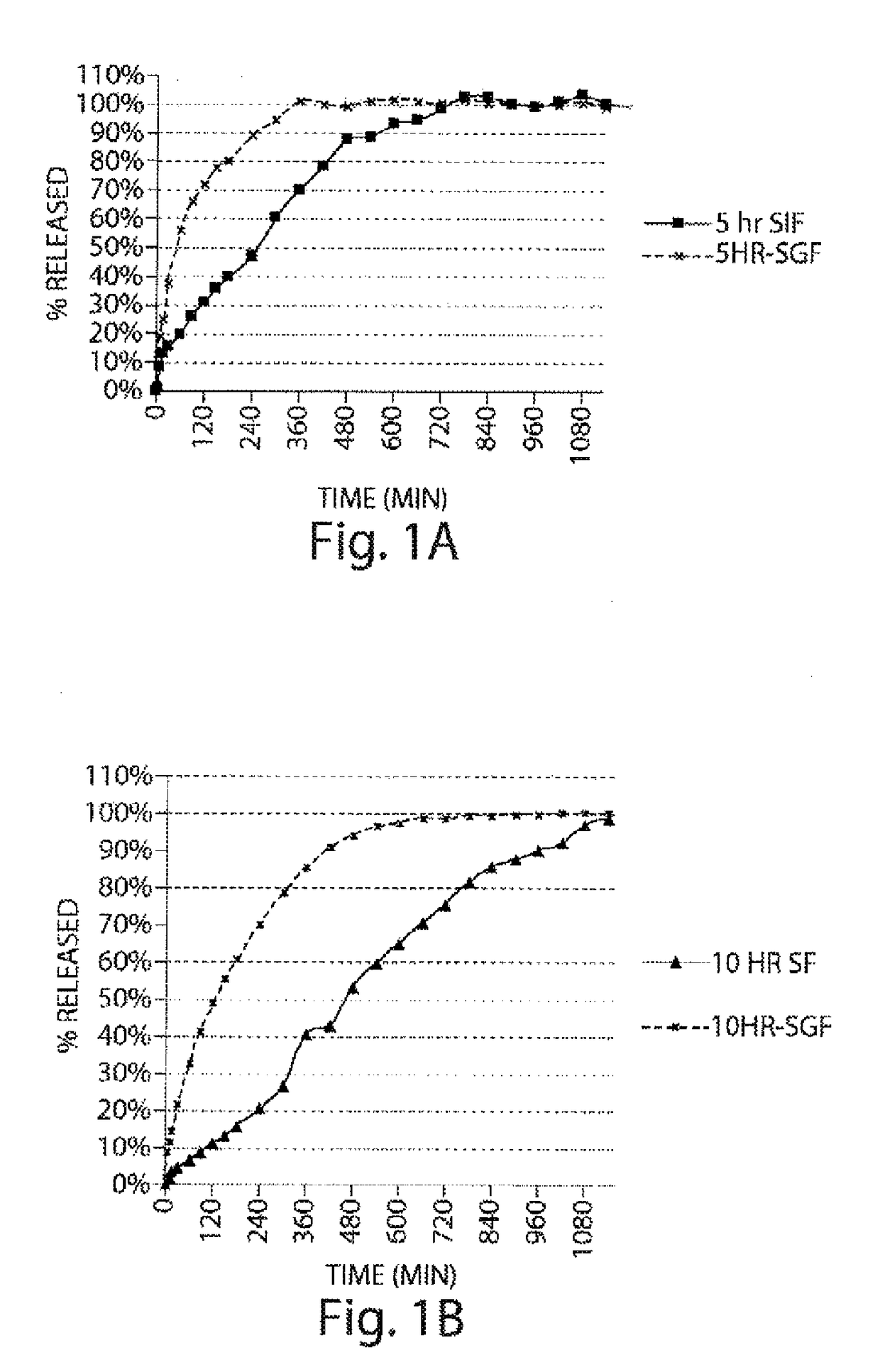
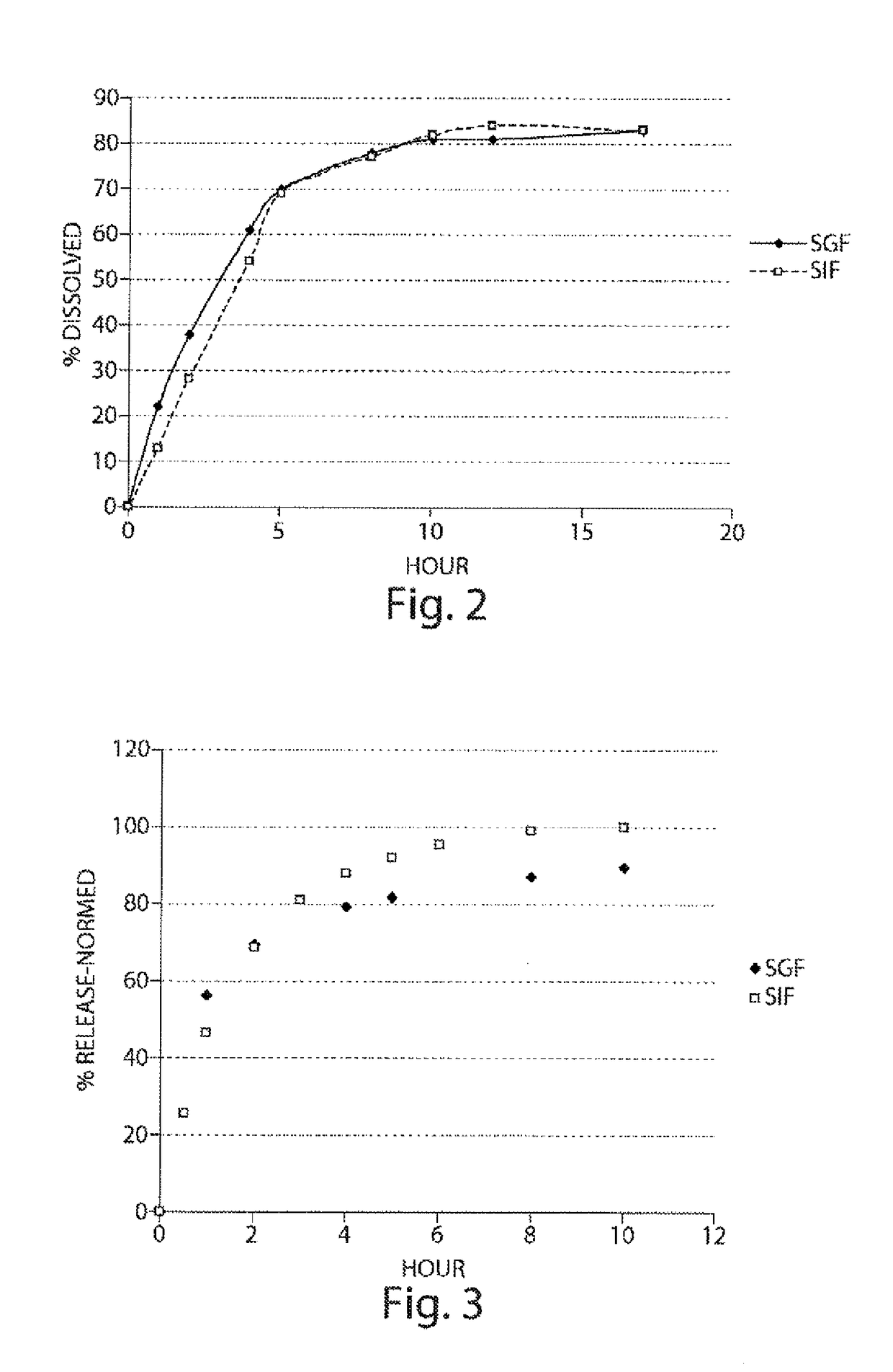
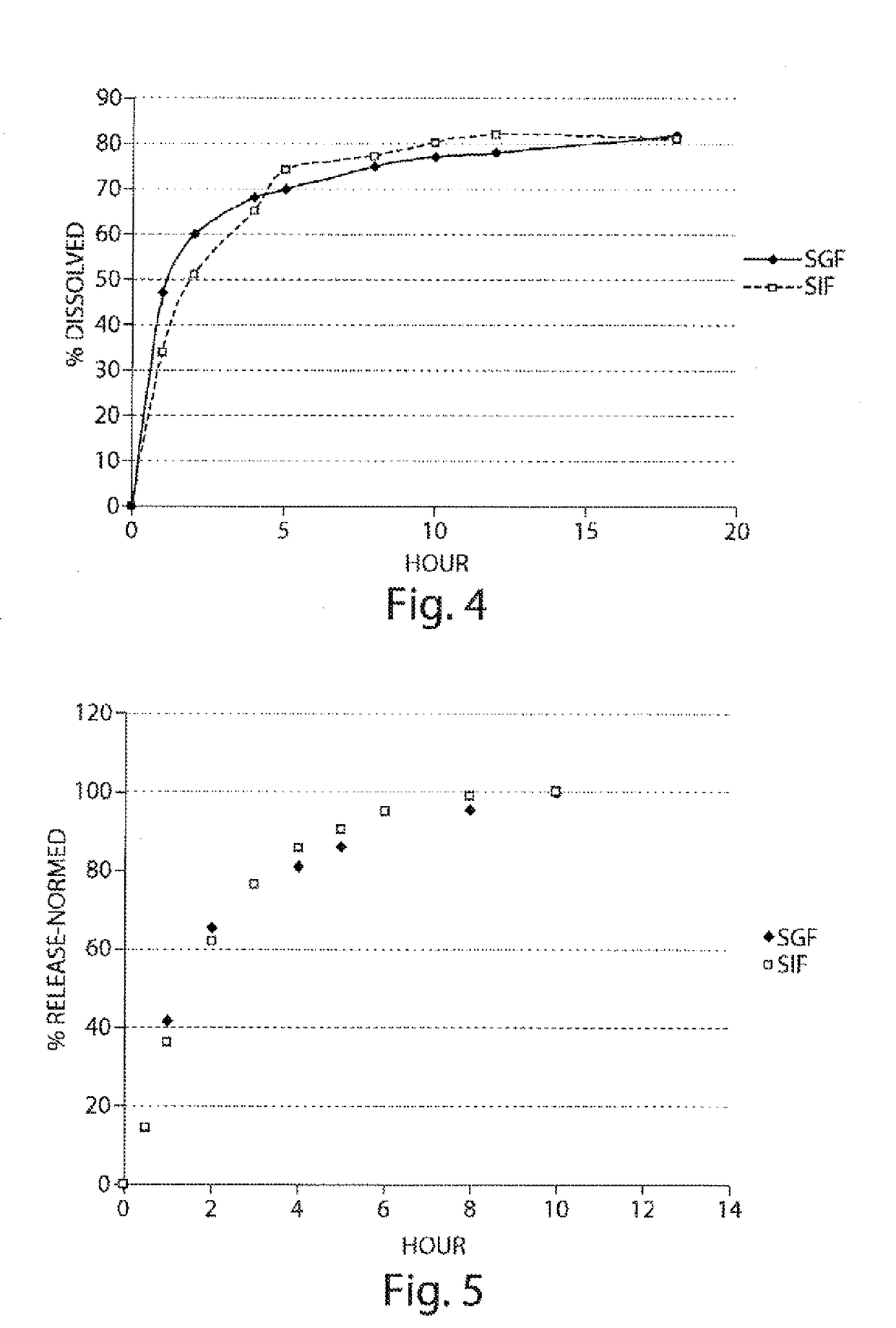
Pharmaceutical compositions of metabotropic glutamate 5 receptor (mGlu5) antagonists or a pharmacologically acceptable salt thereof are disclosed. The compositions contain the therapeutic active compound with non-ionic polymer and ionic polymer, binder and fillers in either matrix pellet, matrix tablet or coated pellets. The compositions provide a pH-independent in vitro release profile with NMT 70% in one hour, NMT 85% in 4 hour, and NLT 80% in 8 hours. The compositions are useful for the treatment of CNS disorders, such as Treatment-Resistant Depression (TRD) and Fragile X Syndrome.
Owner:CHATTERJI ASHISH +5
Method for the treatment of depression
The present invention is directed to method for the treatment of depression, for example, treatment resistant depression; wherein the treatment regimen is adjusted depending on the patient's genotype at SNP rs4306882.
Owner:JANSSEN PHARMA NV
Ketamine and cytochrome p 450 inhibitor combinations
InactiveUS20180256534A1Avoid degradationInhibit metabolismOrganic active ingredientsNervous disorderCytochrome p450 enzymeTreatment resistant
Compositions and methods of treating depression infections are provided. More particularly, compositions including a combination of ketamine and a cytochrome p450 enzyme inhibitor are provided. Methods of using the compositions for treatment of depression, including treatment-resistant or treatment-refractory depression, are provided.Compositions and methods of treating depression infections are provided. More particularly, compositions including a combination of ketamine and a cytochrome p450 enzyme inhibitor are provided. Methods of using the compositions for treatment of depression, including treatment-resistant or treatment-refractory depression, are provided.
Owner:SEQUOIA PHARMACEUTICALS INC
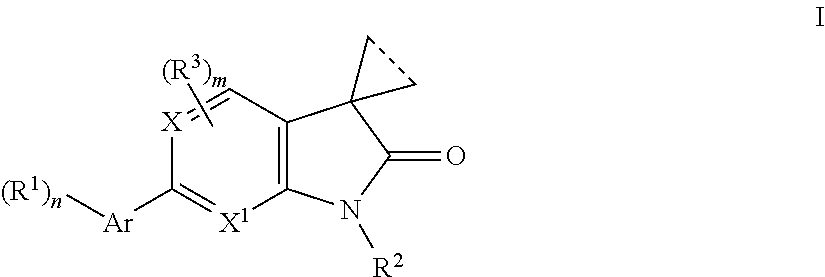
Indolin-2-one or pyrrolo-pyridin/pyrimidin-2-one derivatives
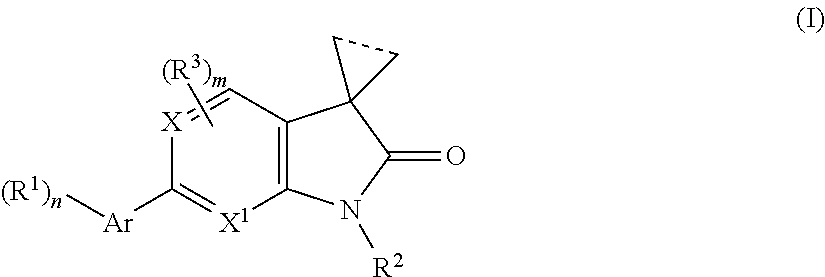
The present invention is concerned with 2-oxo-2,3-dihydro-indoles of general formulawhereinAr is a 6-membered heteroaryl group, containing one or two N-atoms, which are the groups, pyridinyl, pyrimidinyl, pyridazinyl, or a 5-membered heteroaryl group containing from 1 to 3 heteroatoms, selected from N, S or O, which groups are imidazolyl, 1,2,4-oxadiazolyl, 1,3,4-oxadazolyl, isoxazolyl, oxazolyl, 1,3,4-thiadiazolyl or pyrazolyl;R1 is hydrogen, lower alkyl, halogen, amino, dimethylamino, cyano, lower alkyl substituted by halogen, lower alkyl substituted by hydroxy, CH(OH)CF3, (CH2)o-lower alkoxy, cycloalkyl optionally substituted by CF3, or heterocycloalkyl optionally substituted by lower alkyl;R2 is hydrogen, lower alkyl, (CH2)o-cycloalkyl, (CH2)o—O-cycloalkyl, (CH2)o-lower alkoxy, CH2)o-lower alkoxy substituted by halogen, (CH2)o-heterocycloalkyl optionally substituted by lower alkyl, (CH2)o—S(O)2-cycloalkyl, lower alkyl substituted by one or two hydroxy, lower alkyl substituted by one or two lower alkoxy, (CH2)o—S(O)2-lower alkyl, lower alkyl substituted by halogen or CH2CH(OH)CF3;R3 is halogen or lower alkyl;X is CH or N;X1 is CH or N;n is 1 or 2;is 0, 1, 2 or 3;m is 0, 1 or 2;and the dotted line indicates a bond may or may not be present; or,a pharmaceutically acceptable salts thereof, with a racemic mixture, or with its corresponding enantiomer and / or optical isomer and / or stereoisomer thereofThe compounds may be used for the treatment of certain central nervous system disorders which are positive (psychosis) and negative symptoms of schizophrenia, substance abuse, alcohol and drug addiction, obsessive-compulsive disorders, cognitive impairment, bipolar disorders, mood disorders, major depression, treatment resistant depression, anxiety disorders, Alzheimer's disease, autism, Parkinson's disease, chronic pain, borderline personality disorder, sleep disturbances, chronic fatigue syndrome, stiffness, antiinflammatory effects in arthritis and balance problems.
Owner:F HOFFMANN LA ROCHE & CO AG
Kit and method for detecting genes related to individualized medication for depression
PendingCN110157793APredict efficacyPredictive reactivityMicrobiological testing/measurementDNA/RNA fragmentationDrug metabolismNucleotide
The invention discloses a primer group, a kit and a detection method for detecting genes related to individualized medication for depression. The primer group is selected from nucleotide sequences inSEQ ID NO.1-SEQ ID NO.154. The detection kit covers eight major categories of clinical first-line and second-line antidepressant efficacy-related genes, and relates to drug metabolism, target spots and transporter gene loci, and detection is conducted comprehensively and accurately. Accordingly, aiming at part of patients suffering from treatment-refractory depression, gene detection related to the therapeutic effect of antidepressant synergistic drugs is added, and powerful support is provided for selection of therapeutic schedules of the treatment-refractory depression.
Owner:广州海思医疗科技有限公司
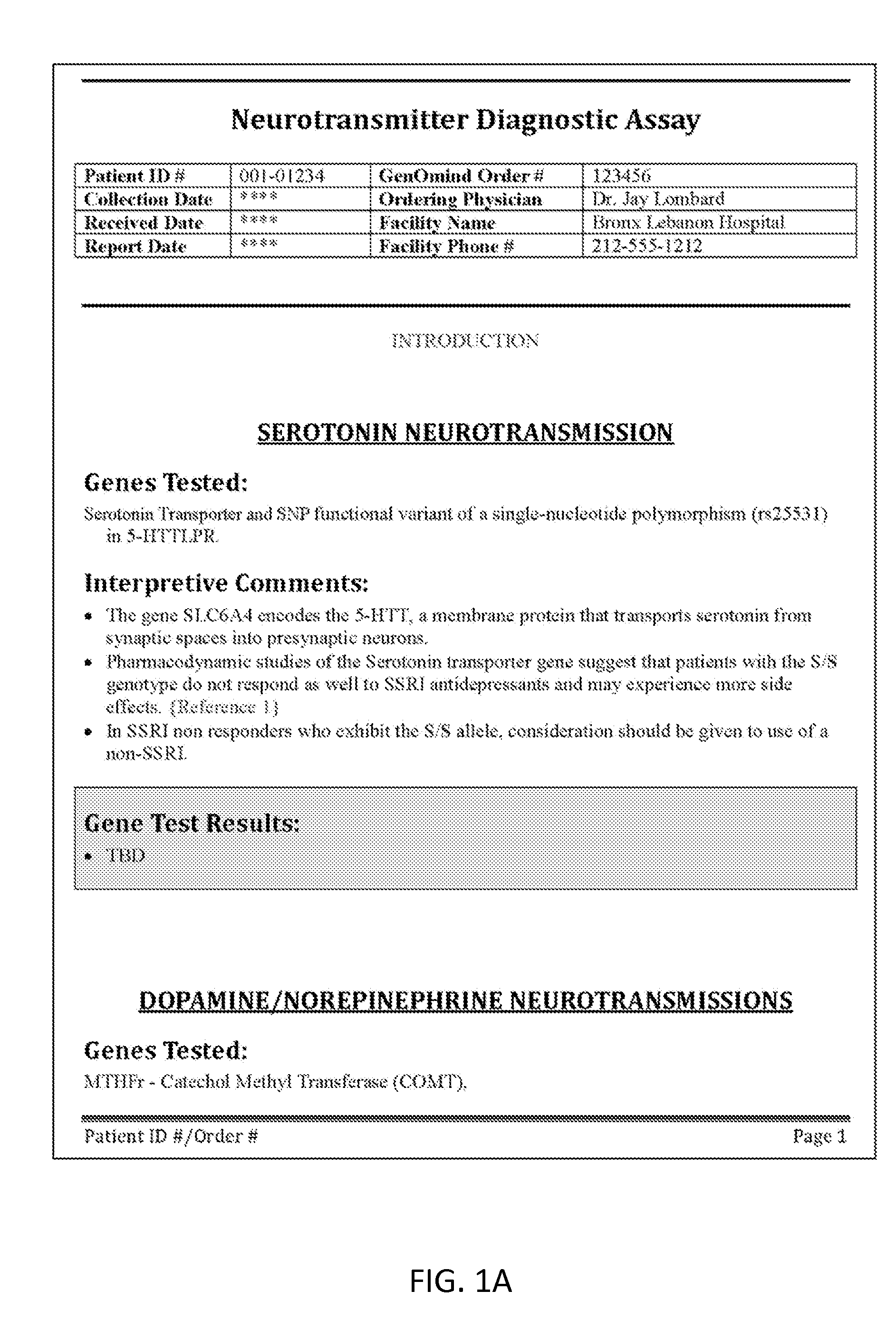
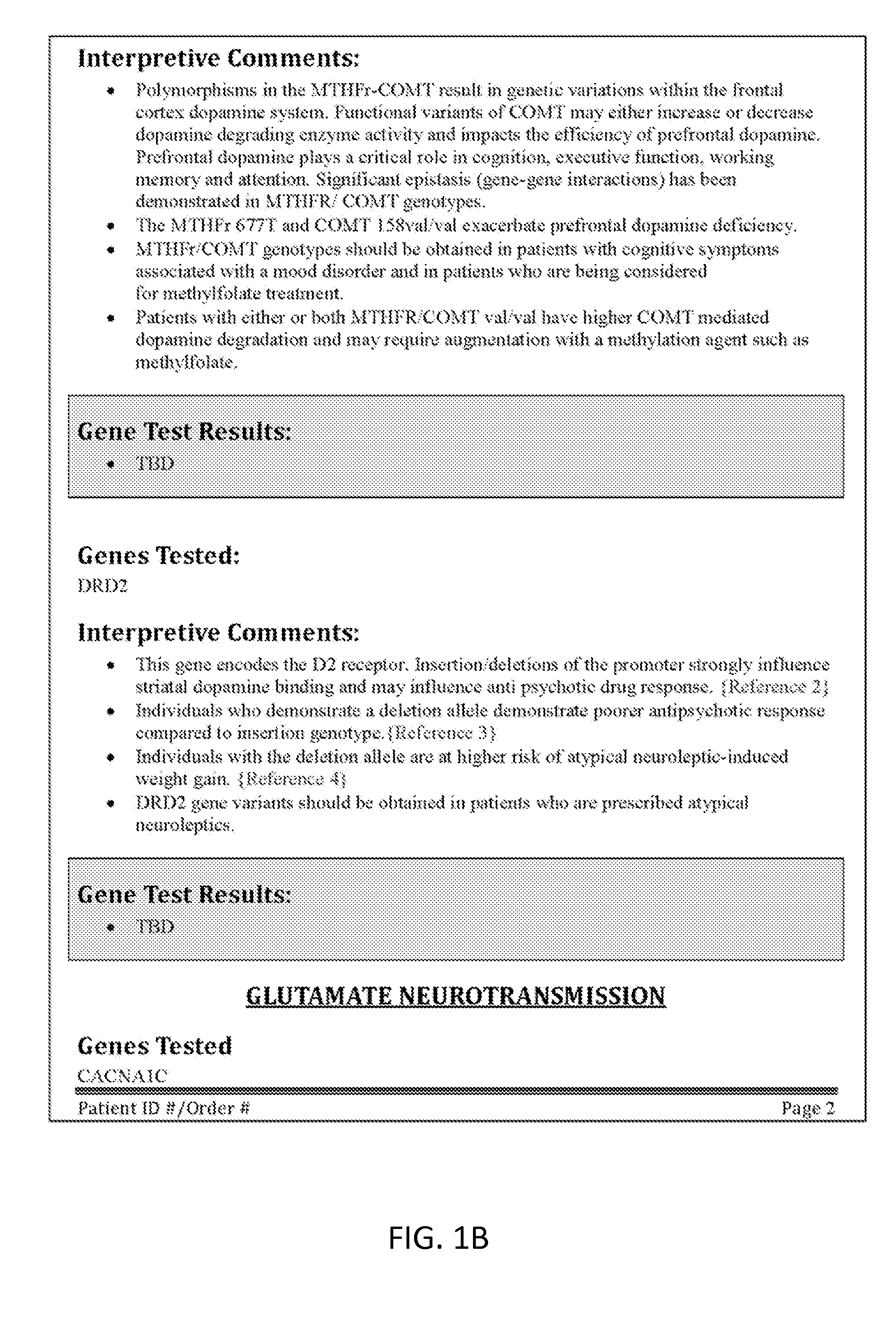
Neuropsychiatric test reports
InactiveUS20120115147A1Enhance diagnostic certaintyCost effectiveData processing applicationsHealth-index calculationDiseaseGenotype
Methods and reports for presenting genetic information that is patient-specific and relevant to treatment of neuropsychiatric disorders, including treatment resistant depression. The methods and reports described include genotype information for each of six specific genetic loci and allow patient-specific therapy for the effective treatment of treatment resistant disorders (TRD).
Owner:GENOMIND
Method for the treatment of depression
The present invention is directed to method for the treatment of depression, for example, treatment resistant depression; wherein the treatment regimen is adjusted depending on the patient's genotype at SNP rs4306882.
Owner:JANSSEN PHARMA NV
Pharmaceutical compositions of metabotropic glutamate 5 receptor (MGLU5) antagonists
Pharmaceutical compositions of metabotropic glutamate 5 receptor (mGlu5) antagonists or a pharmacologically acceptable salt thereof are disclosed. The compositions contain the therapeutic active compound with non-ionic polymer and ionic polymer, binder and fillers in either matrix pellet, matrix tablet or coated pellets. The compositions provide a pH-independent in vitro release profile with NMT 70% in one hour, NMT 85% in 4 hour, and NLT 80% in 8 hours. The compositions are useful for the treatment of CNS disorders, such as Treatment-Resistant Depression (TRD) and Fragile X Syndrome.
Owner:CHATTERJI ASHISH +5
Combined medicine for curing treatment-resistant depression and applications thereof
InactiveCN102133216AHigh remission rateImprove toleranceOrganic active ingredientsNervous disorderDiseaseTolerability
The invention relates to a combined medicine for curing treatment-resistant depression (TRD), which is characterized in that the combined medicine comprises paroxetine and sodium valproate. The invention also provides applications of the combined medicine. The invention has the advantages that the research on the TRD synergia strategy is provided for the first time in China, the combined medicine of paroxetine and sodium valproate, and the applications of the combined medicine in the preparation of medicine for curing the TRD are provided for the first time, the paroxetine combined with the sodium valproate for curing the TRD has higher remission rate, and the tolerance is high, so that the combined medicine can be taken as the new intensive treatment scheme for curing the TRD.
Owner:SHANGHAI MENTAL HEALTH CENT (SHANGHAI PSYCHOLOGICAL COUNSELLING TRAINING CENT)
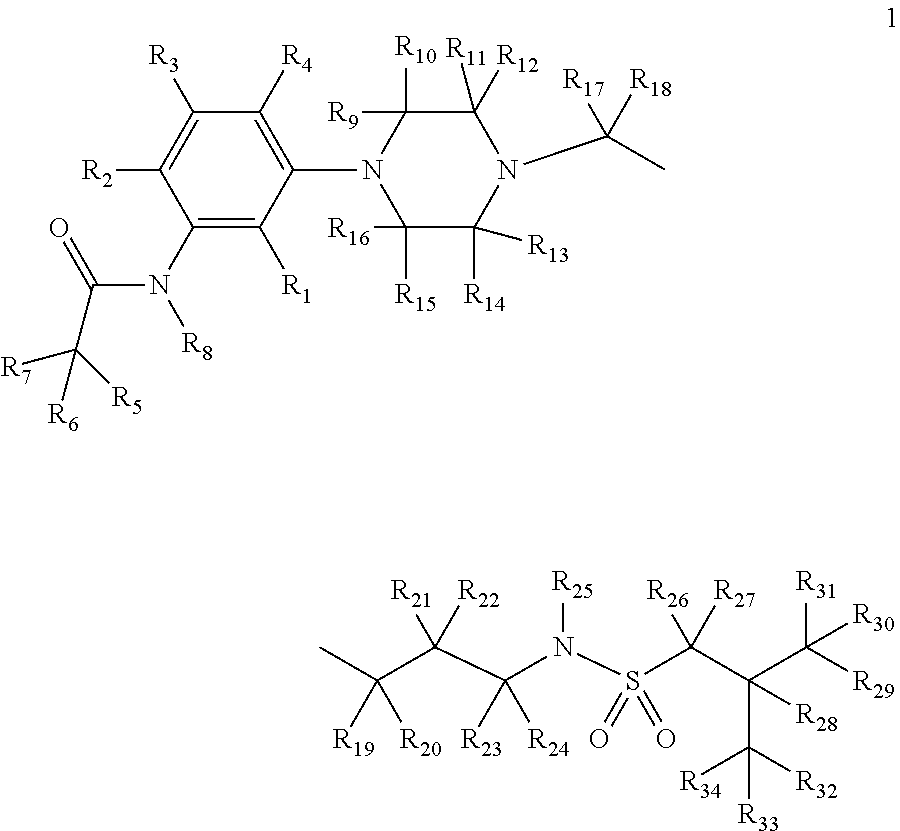
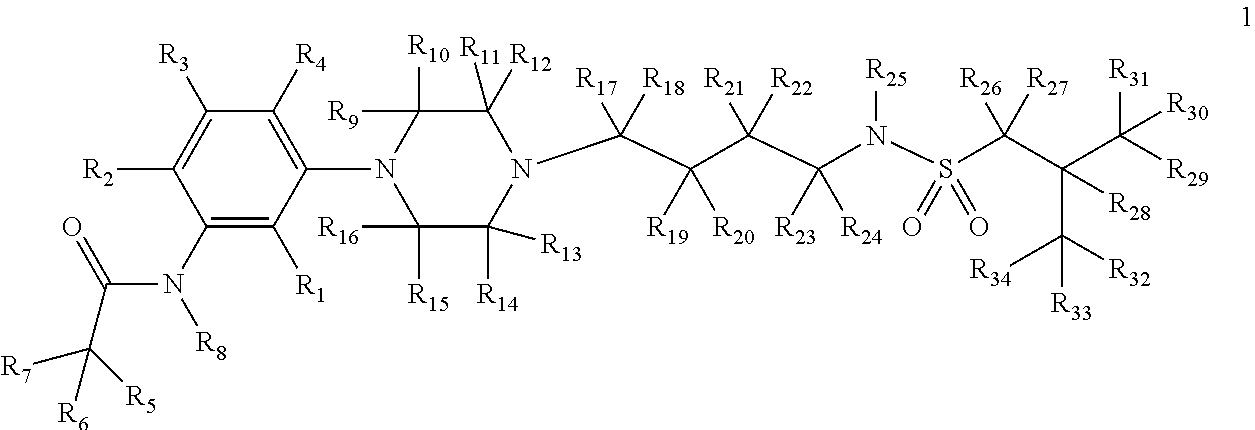
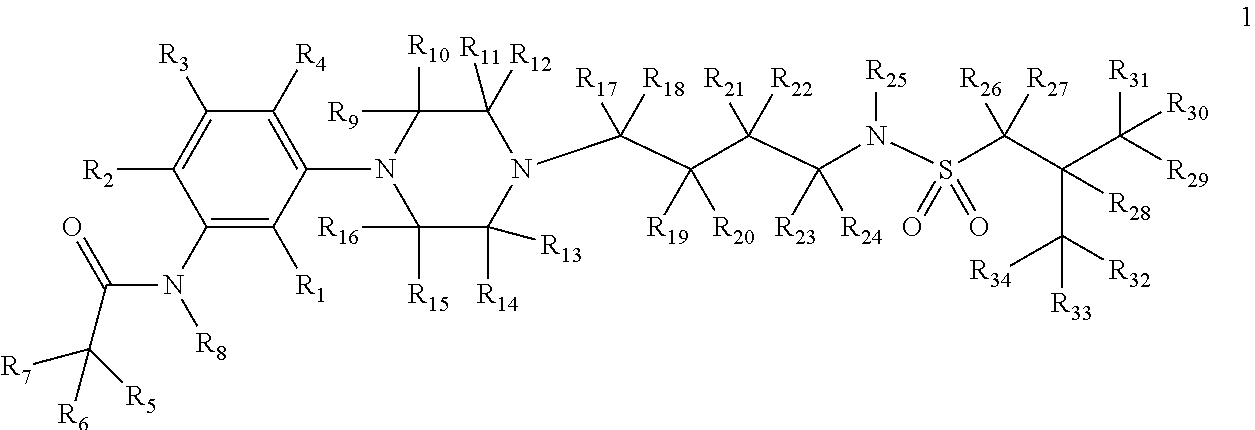
Deuterium-enriched alkyl sulfonamides and uses thereof
ActiveUS8927553B2Prevent curingOrganic active ingredientsOrganic chemistryRecurrent major depressive episodesFibromyalgia
The present invention is concerned with deuterium-enriched sulfonamides of formula 1, their pharmaceutically acceptable salts and methods of use thereof for the treatment of anxiety disorders including, General Anxiety Disorder (GAD), Panic Disorder (PD), Post-Traumatic Stress Disorder (PTSD), Social Phobia (SP), Health Anxiety (Hypochondriasis), depression, major depressive disorders, unipolar depression, bipolar I depression disorder, bipolar II depression disorder, treatment-resistant depression, single episodic and recurrent major depressive disorders, depression in the medically ill, attention deficit hyperactivity disorder (ADHD), attention deficit disorder (ADD), Obsessive-Compulsive Disorder (OCD), Obsessive-Compulsive Personality Disorder (OCPD), Autism Spectrum Disorder (ASD), schizophrenia, psychosis, epilepsy, seizures, hot flashes due to menopause, age-related macular degeneration (AMD), premature ejaculation, male erectile dysfunction, sexual dysfunction, obesity, eating disorders, bulimia nervosa, anorexia nervosa, angina, migraine, pain, nociception, sleep disorders, insomnia, fibromyalgia, alcohol withdrawal, autism, Rett's syndrome, cyclothymic disorder, neural injury, neurodegenerative diseases, Parkinson's disease, Parkinson's disease psychosis, Huntington disease, Alzheimer's disease, frontotemporal dementia, cognitive impairment associated with age-related dementia, Alzheimer's disease, schizophrenia, psychosis, depression, pain or discomfort associated with surgery and pain or discomfort associated with medical illness.
Owner:DHANOA DALJIT SINGH
Treatments For Depression And Other Diseases With A Low Dose Agent
The present invention relates to improved compositions and methods for the treatment or prevention of various diseases, including forms of depression, including, for example, breakthrough depression and treatment-refractory depression, and other mood disorders, as well Parkinson's disease, bipolar disorder, bipolar disorder, attention deficit disorder (ADHD), Restless Leg Syndrome (RLS), and obesity. In some embodiments, the compositions and methods comprise low dose naltrexone or related opioid antagonists.
Owner:PHARMORX THERAPEUTICS
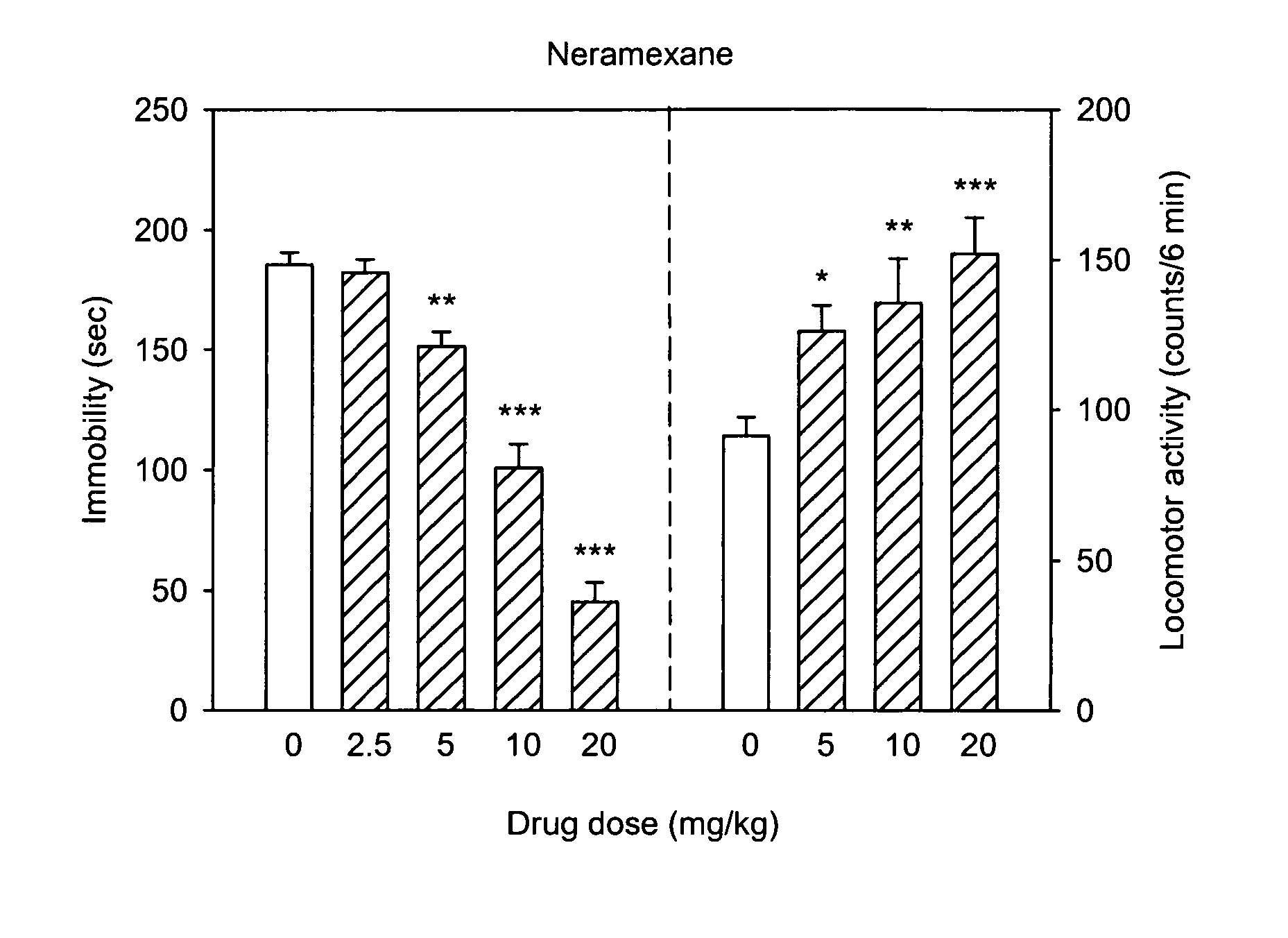
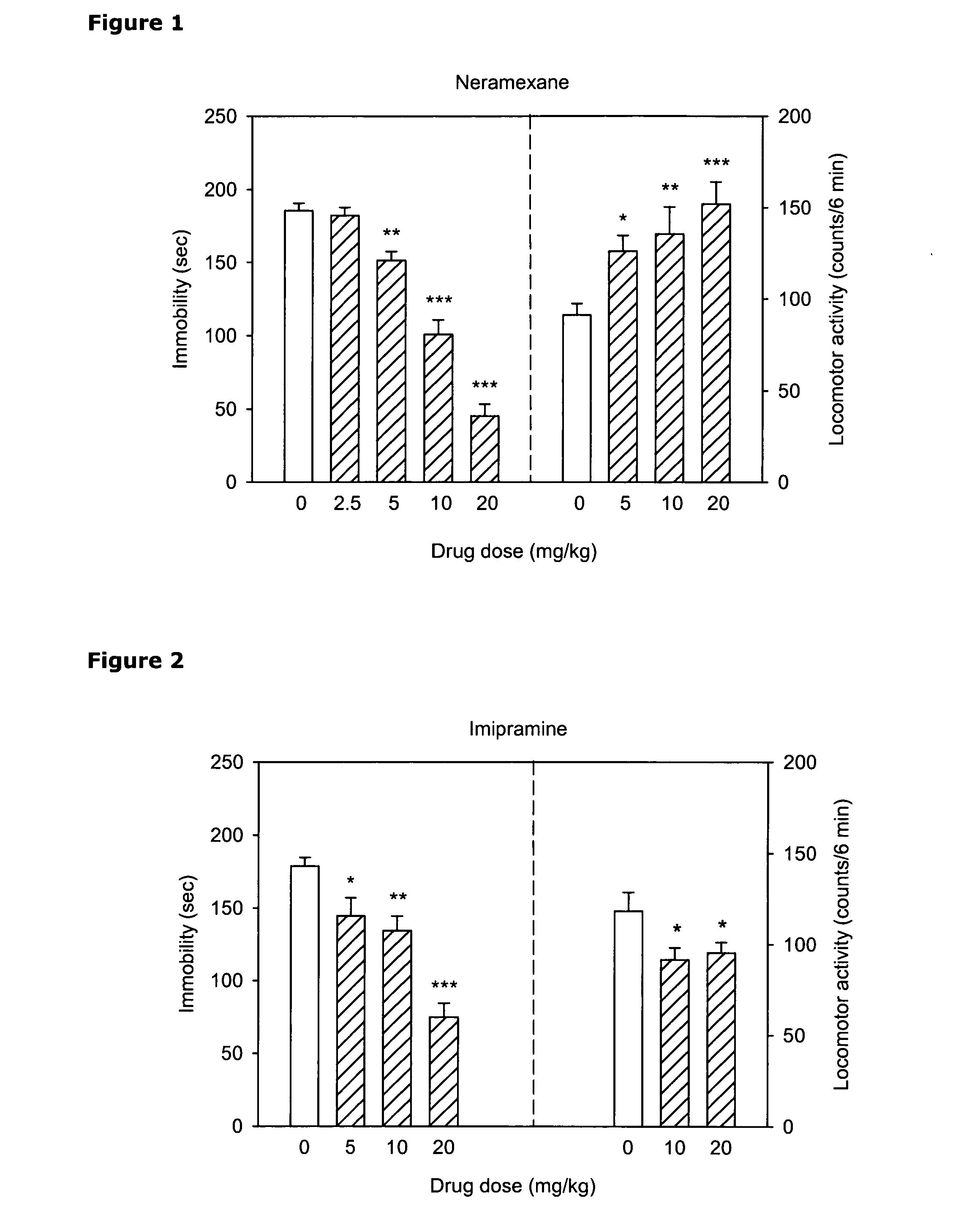
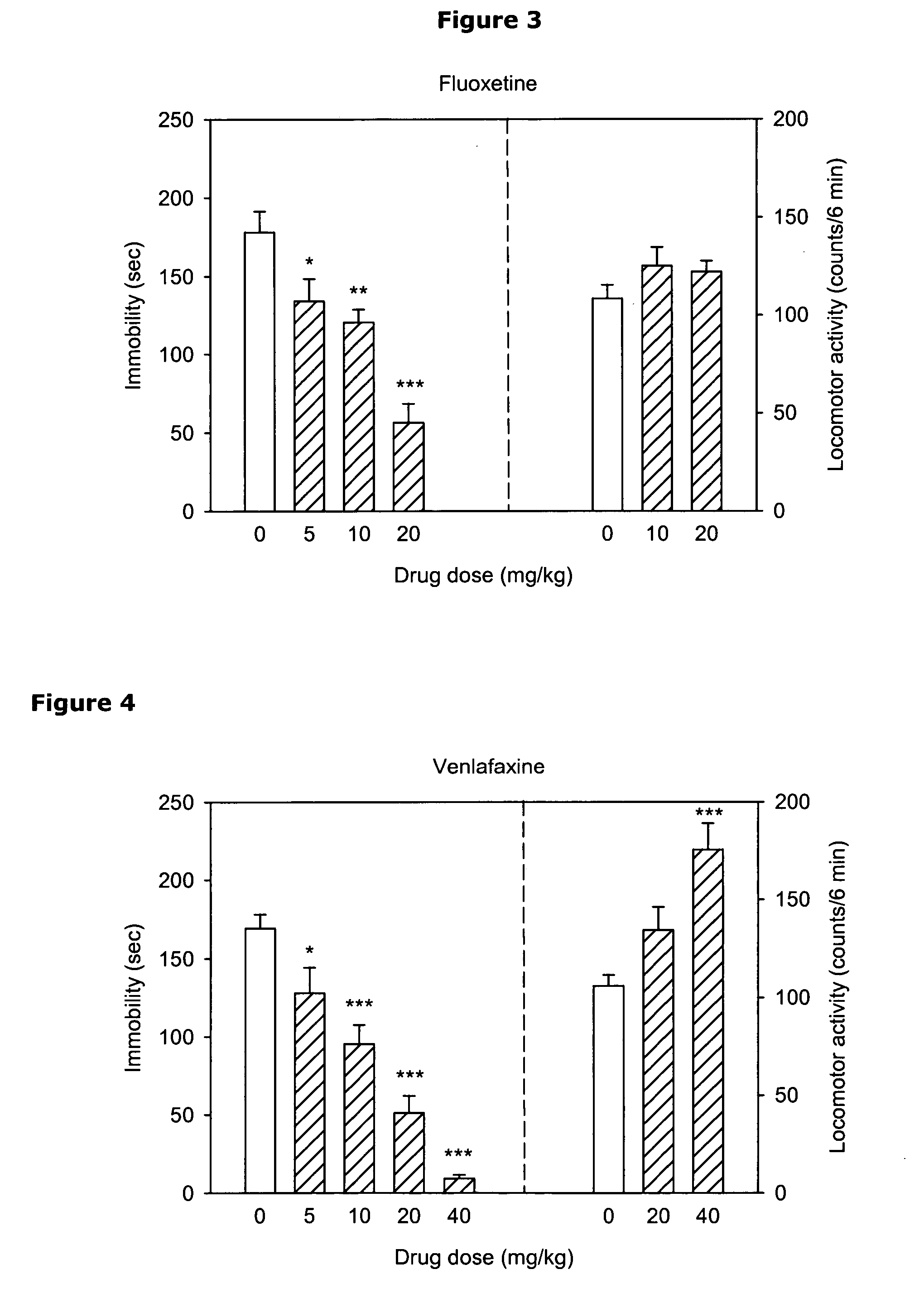
Treatment for therapy refractory depression
A method treats patient with treatment resistant depression, adjunct to standard antidepressant treatment or as monotherapy. These patients will be identified by non-response to at least one pharmacological antidepressant treatment with a sufficient dose and over a sufficient period of time. Further, one embodiment of the method includes a procedure to identify patients who would benefit from a combination of standard antidepressants with the described compounds from the start of the treatment in order to maximize the likelihood of response. Another embodiment of the method combines the described compounds with forms of Magnesium salts in order to overcome longer term tolerability issues with the compounds. An important downstream mechanism of the described interventions is the property to reduce the production and or increase the absorption of cerebrospinal fluid to treat a variety of symptoms, including cognitive dysfunction, memory loss, apathy, sleep disturbances, pain disorders, and somatoform pain and headache.
Owner:MURCK HARALD
Combination of an NMDA receptor antagonist and a selective serotonin reuptake inhibitor for the treatment of depression and other mood disorders
The present invention provides a method for the treatment of depression, including treatment-resistant depression, and other mood disorders using a combination of an NMDA receptor antagonist and a SSRI that is citalopram or escitalopram. It has unexpectedly been shown that the combination has a synergistic and potentiated effect of either compound as monotherapy, resulting in an enhanced therapeutic effect at lower doses.
Owner:MERZ PHARMA GMBH & CO KGAA
4-[2,3-Difluoro-6-(2-fluoro-4-methyl-phenylsulfanyl)-phenyl]-piperidine
The compound 4-[2,3-Difluoro-6-(2-fluoro-4-methyl-phenylsulfanyl)-phenyl]-piperidine according to the structure (formula I), and pharmaceutically acceptable salts thereof are provided for the treatment of CNS related disorders, such as: depressive disorder, dysthymic disorder; mood disorder due to a general medical condition; atypical depression; seasonal affective disorder; melancholia; treatment resistant depression; partial responders; depression associated with bipolar disorder, pain, Alzheimer's disease, psychosis, Parkinson's disease, Lewy body disease, Huntington's disease, multiple sclerosis or anxiety; general anxiety disorder, social anxiety disorder, panic attacks; phobia; social phobia, obsessive compulsive disorder; post traumatic stress disorder, acute stress; ADHD; and pain.
Owner:H LUNDBECK AS
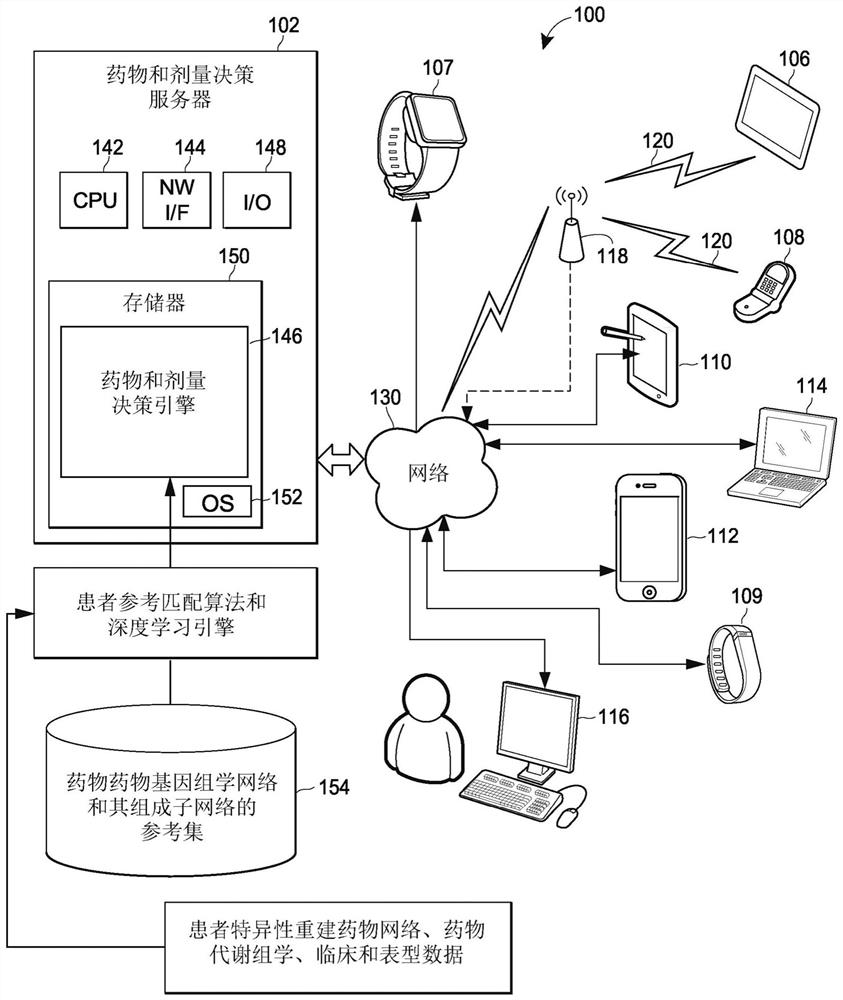
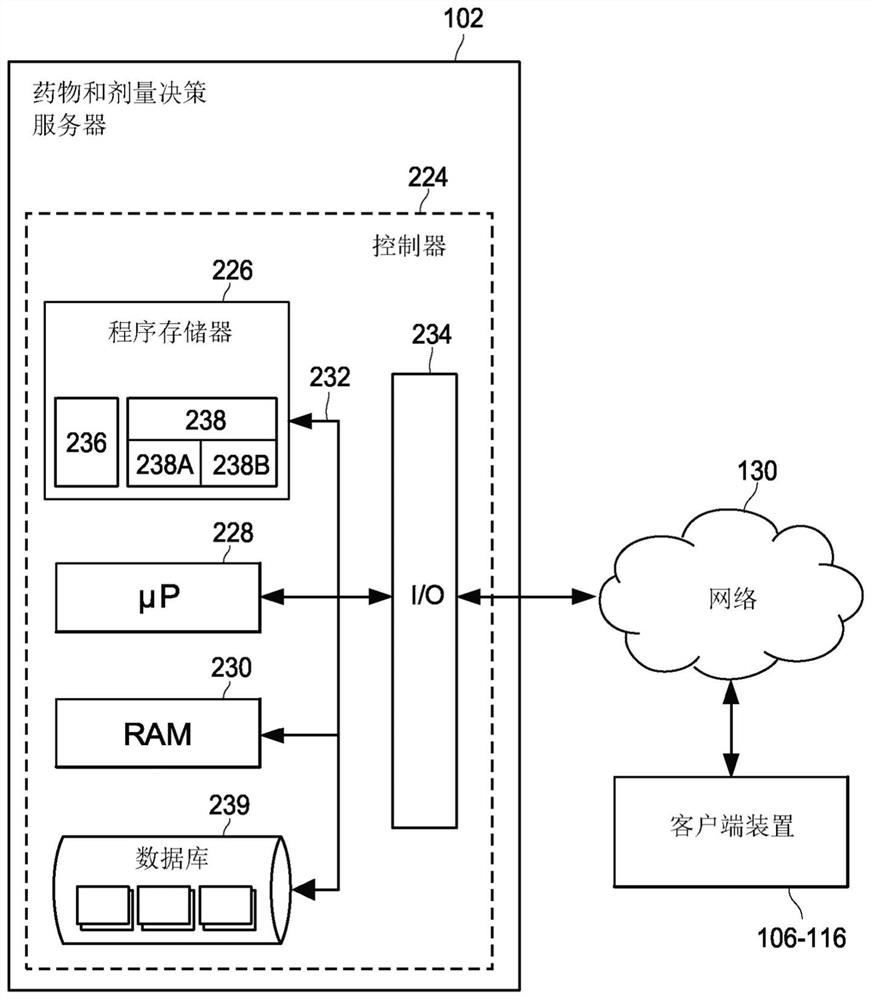
Pharmacogenomic decision support for modulators of the nmda, glycine, and ampa receptors
PendingCN113632174AExtensive computingExtensive Computing SolutionsMedical simulationDrug and medicationsNucleotideEfficacy
Methods for identifying patients diagnosed with treatment resistant or refractory depression, pain or other clinical indications who are eligible to receive N-methyl-D-aspartate receptor antagonist, glycine receptor beta (GLRB) modulator, or alpha-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid receptor (AMPAR)-based therapies to include determining the appropriate medication, an optimal dose for each patient, and determining which patients are not eligible to receive the therapy. The pharmacogenomic clinical decision support assays include targeted single nucleotide polymorphisms and clinical values or a combination of targeted single nucleotide polymorphisms, targeted ketamine- specific expansion and contraction of topologically associated domains, and clinical values. The methods described herein allow for a more effective determination of which patients will experience drug efficacy and which patients will experience adverse drug events. The methods provide personalized patient recommendations for dose, the frequency of medication administration, and recommendations on drug choice.
Owner:RGT UNIV OF MICHIGAN
Pharmaceutical compositions of metabotropic glutamate 5 receptor (MGLU5) antagonists
InactiveUS20170202819A1Organic active ingredientsNervous disorderMetabotropic glutamate receptorPh independent
Pharmaceutical compositions of metabotropic glutamate 5 receptor (mGlu5) antagonists or a pharmacologically acceptable salt thereof are disclosed. The compositions contain the therapeutic active compound with non-ionic polymer and ionic polymer, binder and fillers in either matrix pellet, matrix tablet or coated pellets. The compositions provide a pH-independent in vitro release profile with NMT 70% in one hour, NMT 85% in 4 hour, and NLT 80% in 8 hours. The compositions are useful for the treatment of CNS disorders, such as Treatment-Resistant Depression (TRD) and Fragile X Syndrome.
Owner:F HOFFMANN LA ROCHE & CO AG
Dibenzo[b,f][1,4]oxazapine compounds
The present invention relates to 11-(piperazin-1-yl)dibenzo[b,f][1,4 ]oxazapine compounds of the formula:where the variables are as defined herein, their salts and pharmaceutically acceptable compositions thereof. Methods of preparing these compounds are also described. These compounds may be used in the treatment of disorders such as schizophrenia, treatment resistant schizophrenia, bipolar disorder, psychotic depression, treatment resistant depression, schizophrenia-associated depression, treatment resistant OCD, autism, senile psychosis, psychotic dementia, L-DOPA induced psychosis, psychogenic polydipsia, psychotic symptoms of neurological disorders, sleep disorders.
Owner:PROTOCELL THERAPEUTICS INC
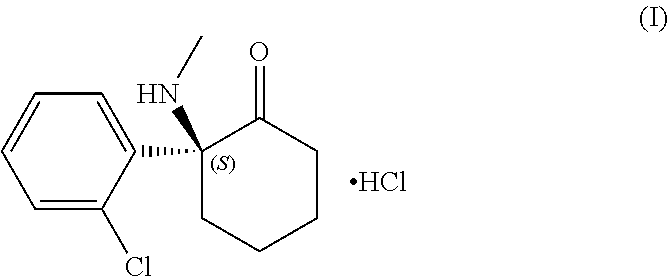
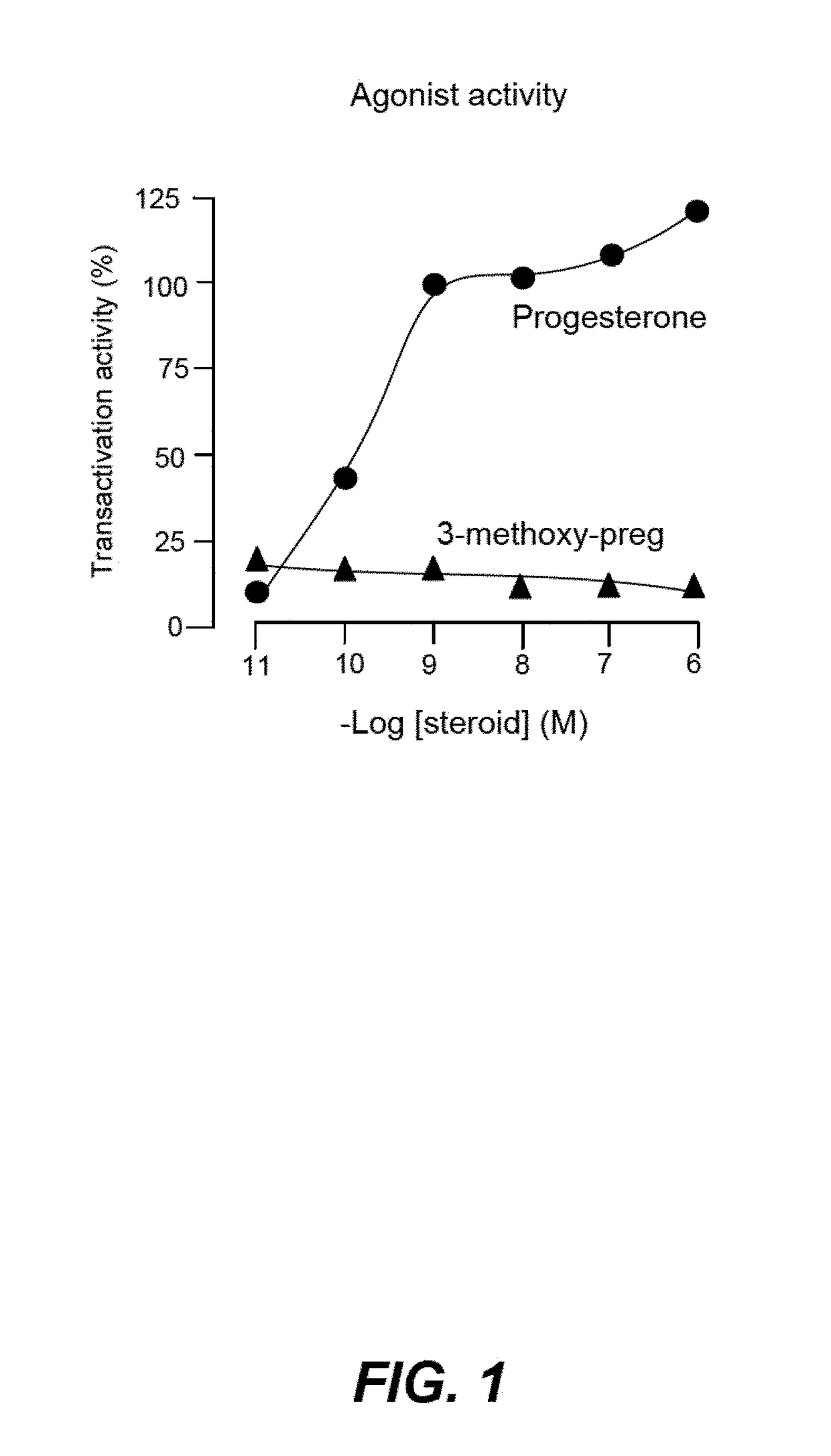
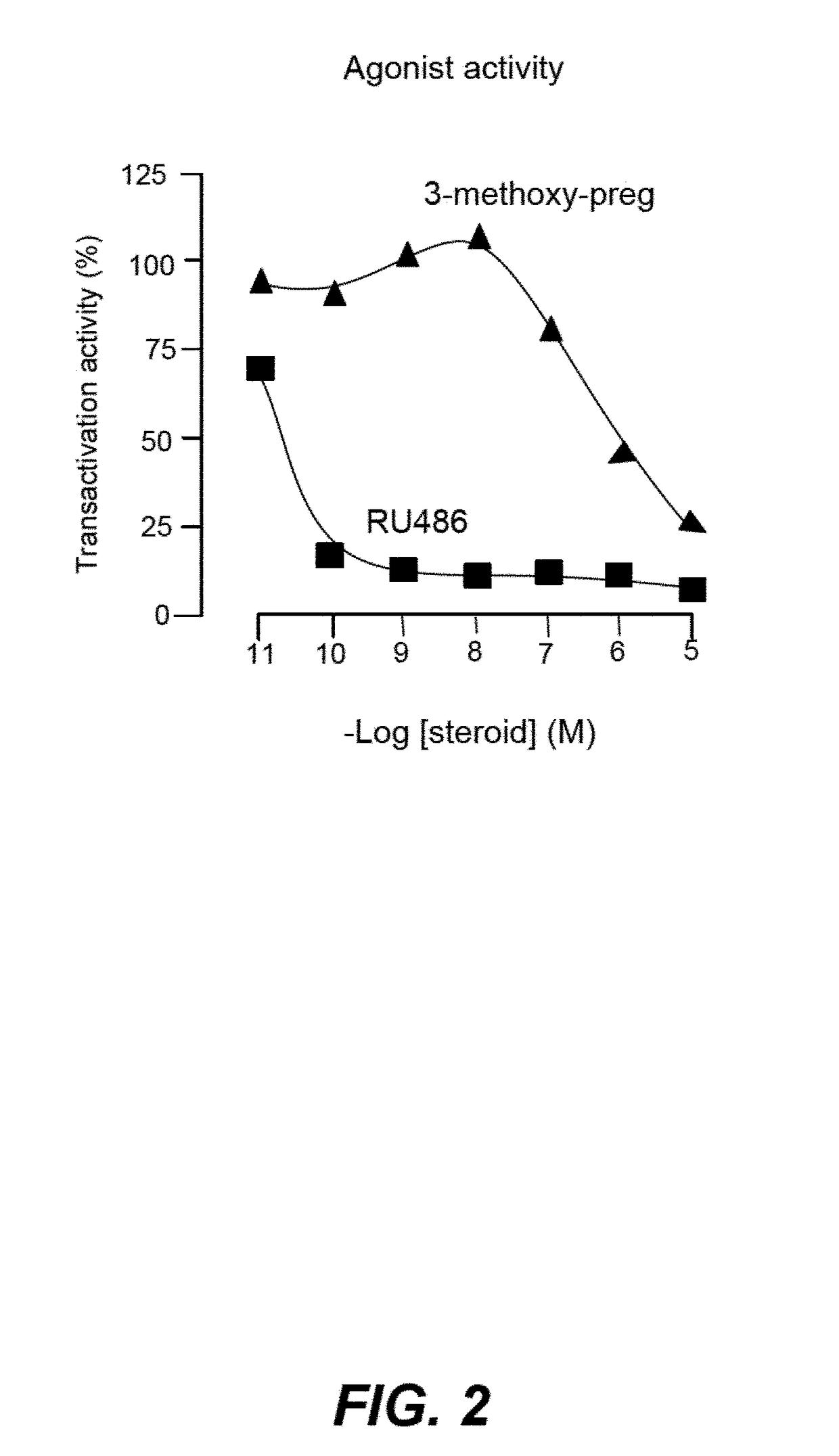
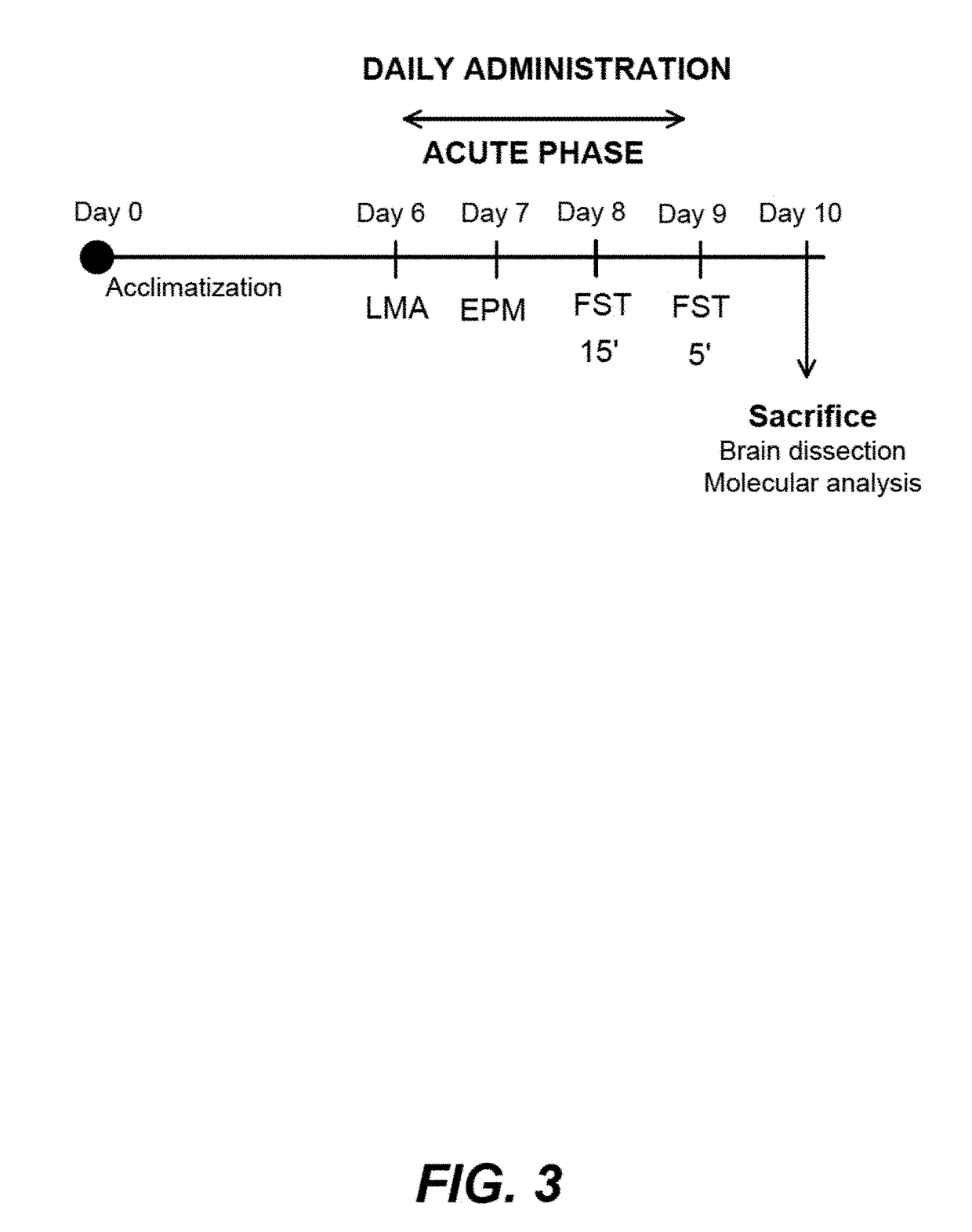
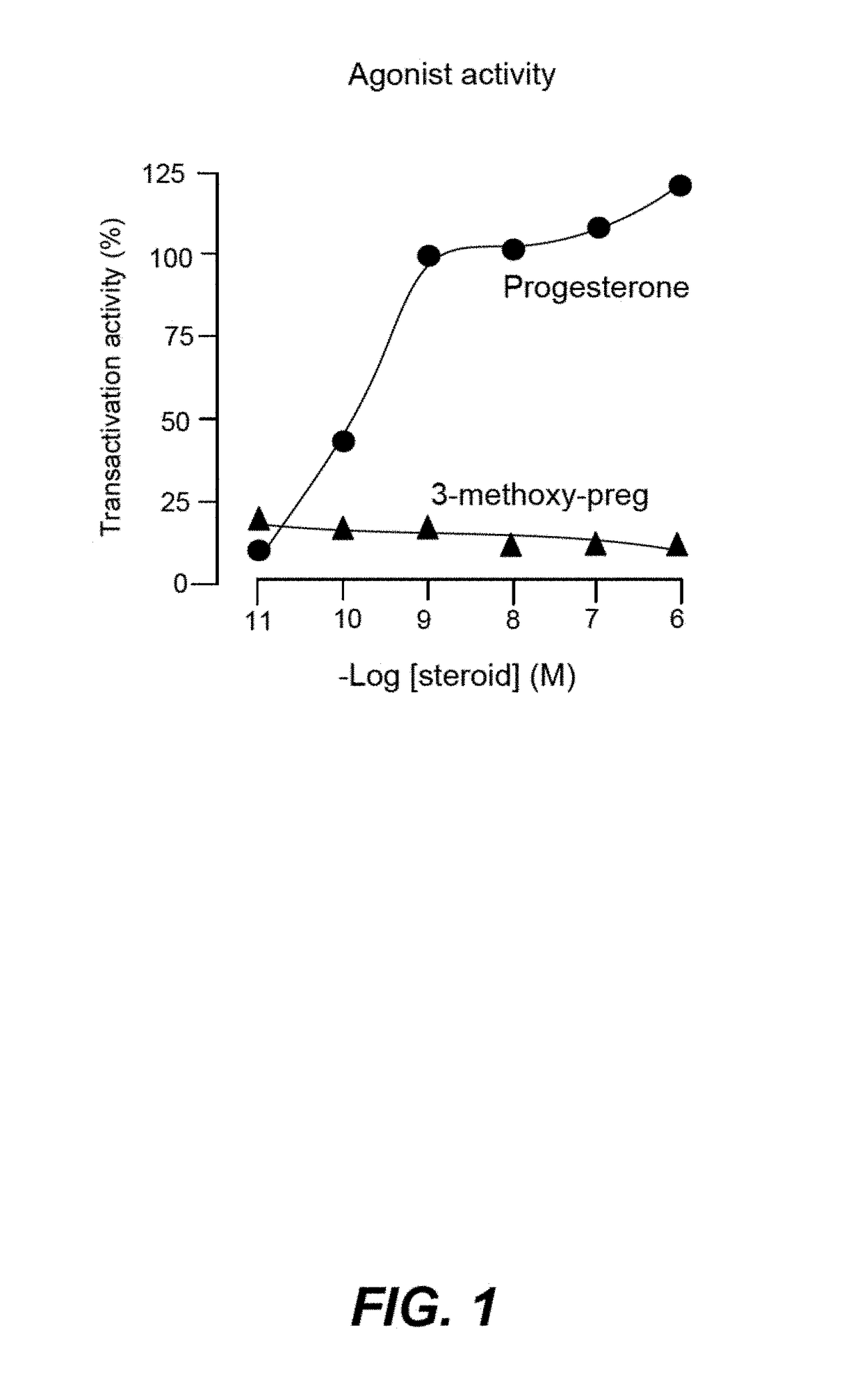
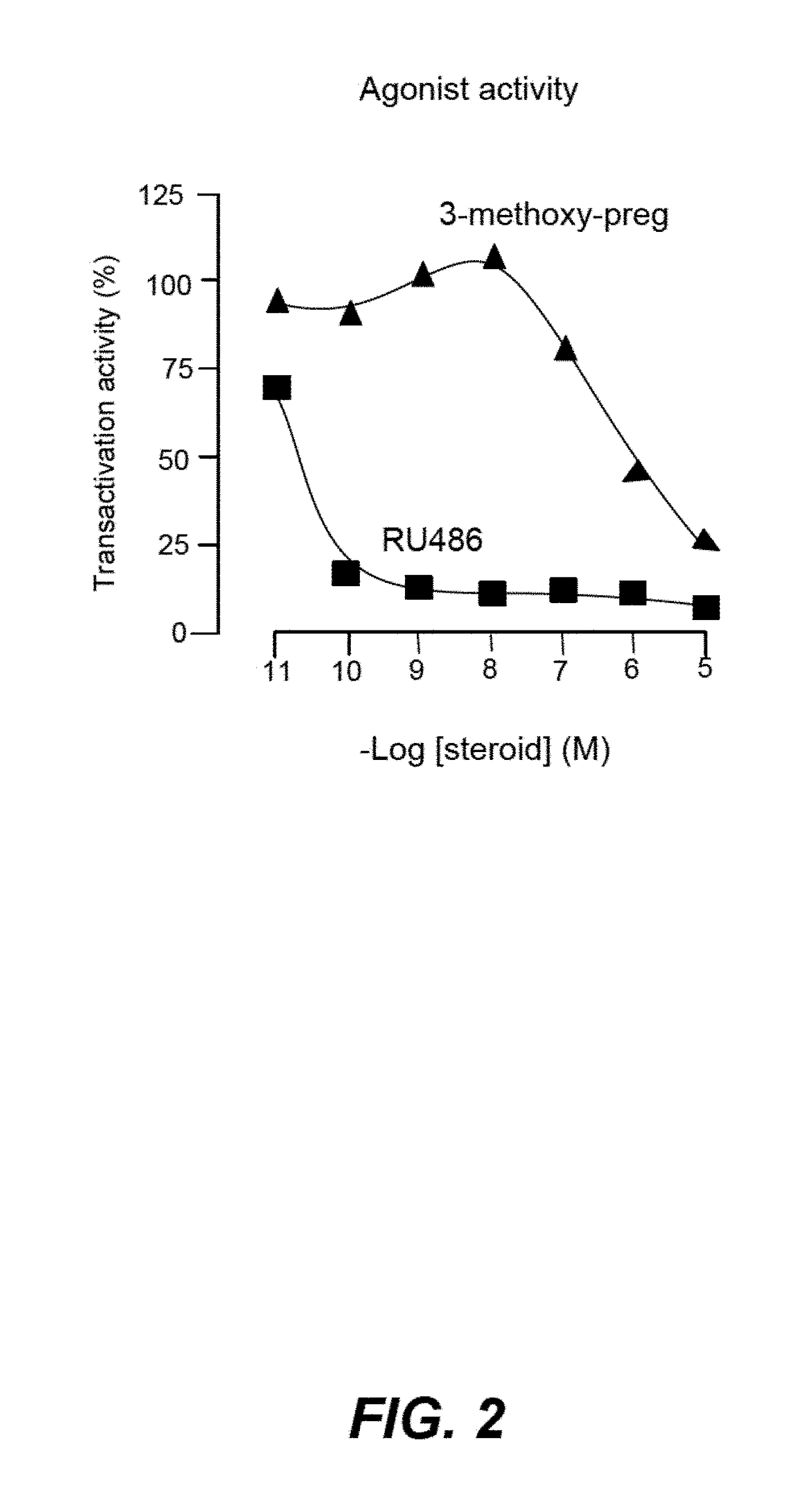
Non-Bioconvertible C3-Substituted Pregnenolone Derivatives for Use in the Treatment of Treatment-Resistant Depression
ActiveUS20170252358A1Reduce depressive symptomImprove the level ofOrganic active ingredientsPregnenoloneSteroid hormone receptor
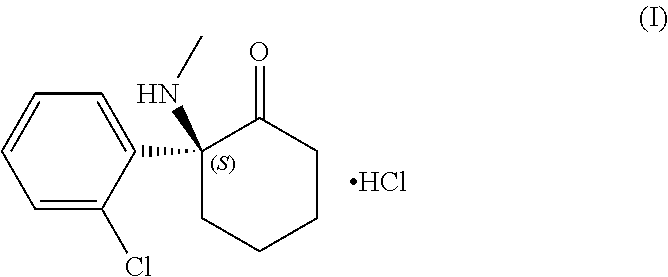
The present invention relates to methods for the treatment of treatment-resistant depression (TRD), comprising the administration of compounds of formula (I), which are blocked in C3 position and cannot metabolize in vivo into pregnenolone derivatives and which do not have significant affinity for steroid hormonal receptors and for all tested classical main receptors and receptors of neurotransmitters of the central nervous system.
Owner:MAPREG
Non-bioconvertible C3-substituted pregnenolone derivatives for use in the treatment of treatment-resistant depression
ActiveUS9943528B2Relieve symptomsSignificantly and rapidly reduce the anxiodepressive-like behaviorOrganic active ingredientsPregnenoloneSteroid hormone receptor
The present invention relates to methods for the treatment of treatment-resistant depression (TRD), comprising the administration of compounds of formula (I), which are blocked in C3 position and cannot metabolize in vivo into pregnenolone derivatives and which do not have significant affinity for steroid hormonal receptors and for all tested classical main receptors and receptors of neurotransmitters of the central nervous system.
Owner:MAPREG
Methods For The Treatment Of Depression
The present invention is directed to methods and dosing regimens for the treatment of depression (preferably, treatment resistant depression), for the treatment of depression in a suicidal patient, and / or for the treatment and / or prevention of suicidality (e.g. suicidal ideations).
Owner:JANSSEN PHARMA NV
Pharmaceutical Compositions Of Metabotropic Glutamate 5 Receptor (MGLU5) Antagonists
Pharmaceutical compositions of metabotropic glutamate 5 receptor (mGlu5) antagonists or a pharmacologically acceptable salt thereof are disclosed. The compositions contain the therapeutic active compound with non-ionic polymer and ionic polymer, binder and fillers in either matrix pellet, matrix tablet or coated pellets. The compositions provide a pH-independent in vitro release profile with NMT 70% in one hour, NMT 85% in 4 hour, and NLT 80% in 8 hours. The compositions are useful for the treatment of CNS disorders, such as Treatment-Resistant Depression (TRD) and Fragile X Syndrome.
Owner:F HOFFMANN LA ROCHE & CO AG
Pharmaceutical formulation for use in the treatment of depressive and anxiety disorders
The present invention is aimed at a novel composition for use as a medicament; it is also aimed at said composition for use in the treatment of depressive and anxiety syndromes. In particular, said composition is for use in the treatment of: major depression, generalized anxiety disorder, social phobia, panic disorder, mixed depression and anxiety disorder, somatoform disorder, treatment-resistant depression, obsessive-compulsive disorder. The invention also relates to a process for the preparation of said pharmaceutical composition.
Owner:TURRI MILO
Pharmaceutical formulation for use in the treatment of depressive and anxiety disorders
Owner:TURRI MILO
DIBENZO[b,f][1,4]OXAZAPINE COMPOUNDS
The present invention relates to 11-(piperazin-1-yl)dibenzo[b,f][1,4]oxazapine compounds of the formula:where the variables are as defined herein, their salts and pharmaceutically acceptable compositions thereof. Methods of preparing these compounds are also described. These compounds may be used in the treatment of disorders such as schizophrenia, treatment resistant schizophrenia, bipolar disorder, psychotic depression, treatment resistant depression, schizophrenia-associated depression, treatment resistant OCD, autism, senile psychosis, psychotic dementia, L-DOPA induced psychosis, psychogenic polydipsia, psychotic symptoms of neurological disorders, sleep disorders.
Owner:PROTOCELL THERAPEUTICS INC
Treatment for Therapy Refractory Depression
A method treats patient with treatment resistant depression, adjunct to standard antidepressant treatment or as monotherapy. These patients will be identified by non-response to at least one pharmacological antidepressant treatment with a sufficient dose and over a sufficient period of time. Further, one embodiment of the method includes a procedure to identify patients who would benefit from a combination of standard antidepressants with the described compounds from the start of the treatment in order to maximize the likelihood of response. Another embodiment of the method combines the described compounds with forms of Magnesium salts in order to overcome longer term tolerability issues with the compounds. An important downstream mechanism of the described interventions is the property to reduce the production and or increase the absorption of cerebrospinal fluid to treat a variety of symptoms, including cognitive dysfunction, memory loss, apathy, sleep disturbances, pain disorders, and somatoform pain and headache.
Owner:MURCK HARALD
Gaboxadol for reducing risk of suicide and rapid relief of depression
PendingCN113395962ARelieves symptoms of depressionNervous disorderOrganic chemistrySUICIDAL TENDENCYRegimen
Methods and compositions are disclosed for rapidly reducing the risk of suicide in patients suffering from acute suieidality and rapidly relieving mood symptoms in major depression and treatment-resistant depression using a novel therapeutic regimen comprising a single or intermittent administration of a high dose of gaboxadol, or a pharmaceutically acceptable salt thereof, to the subject in need thereof.
Owner:CERTEGO THERAPEUTICS
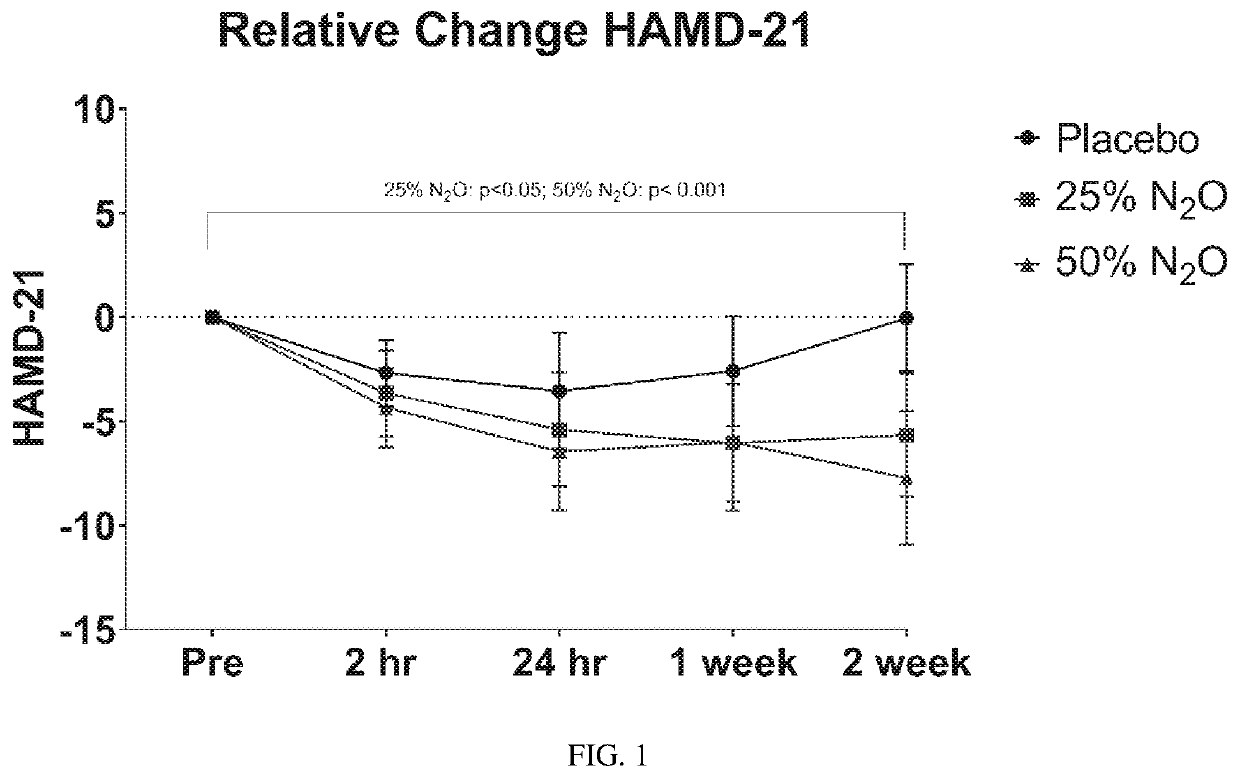
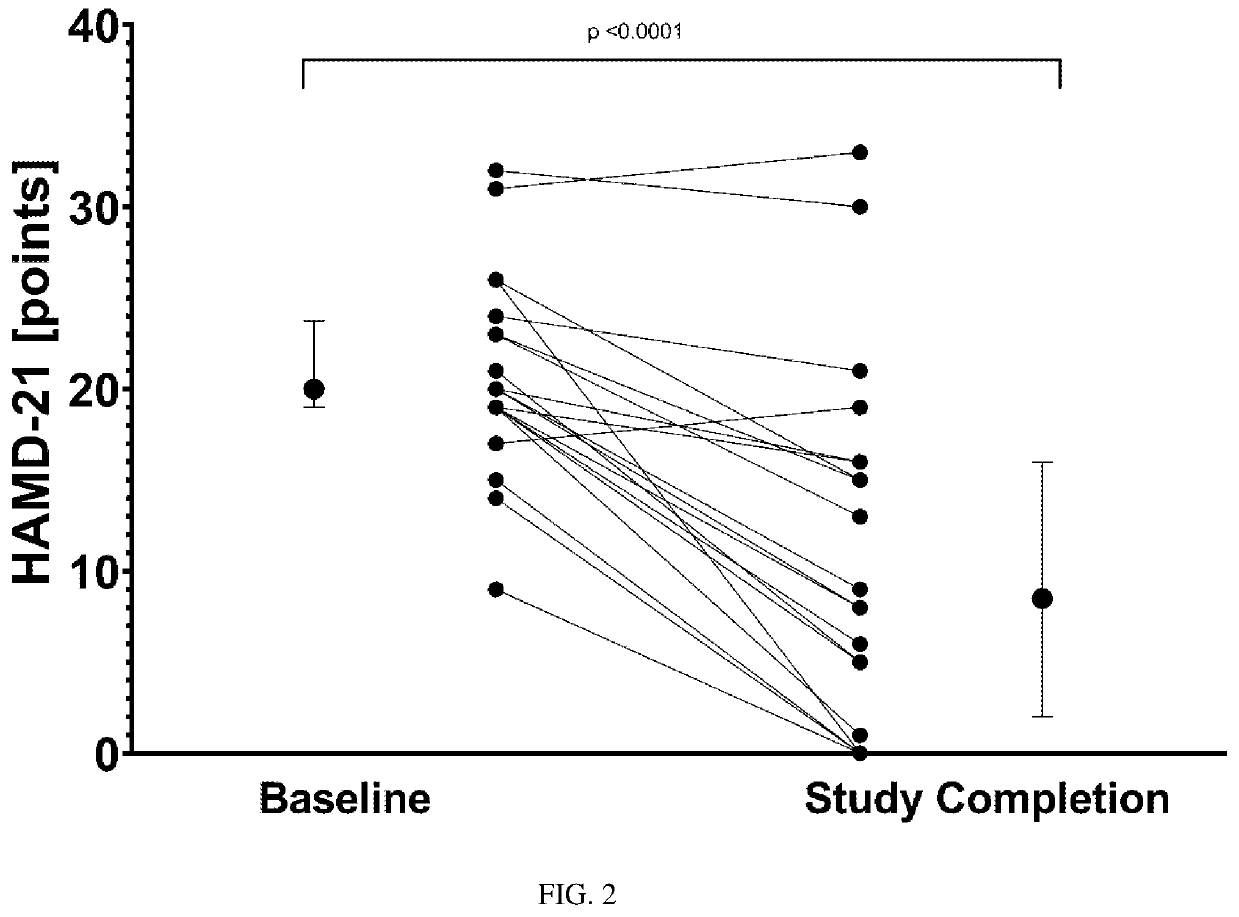
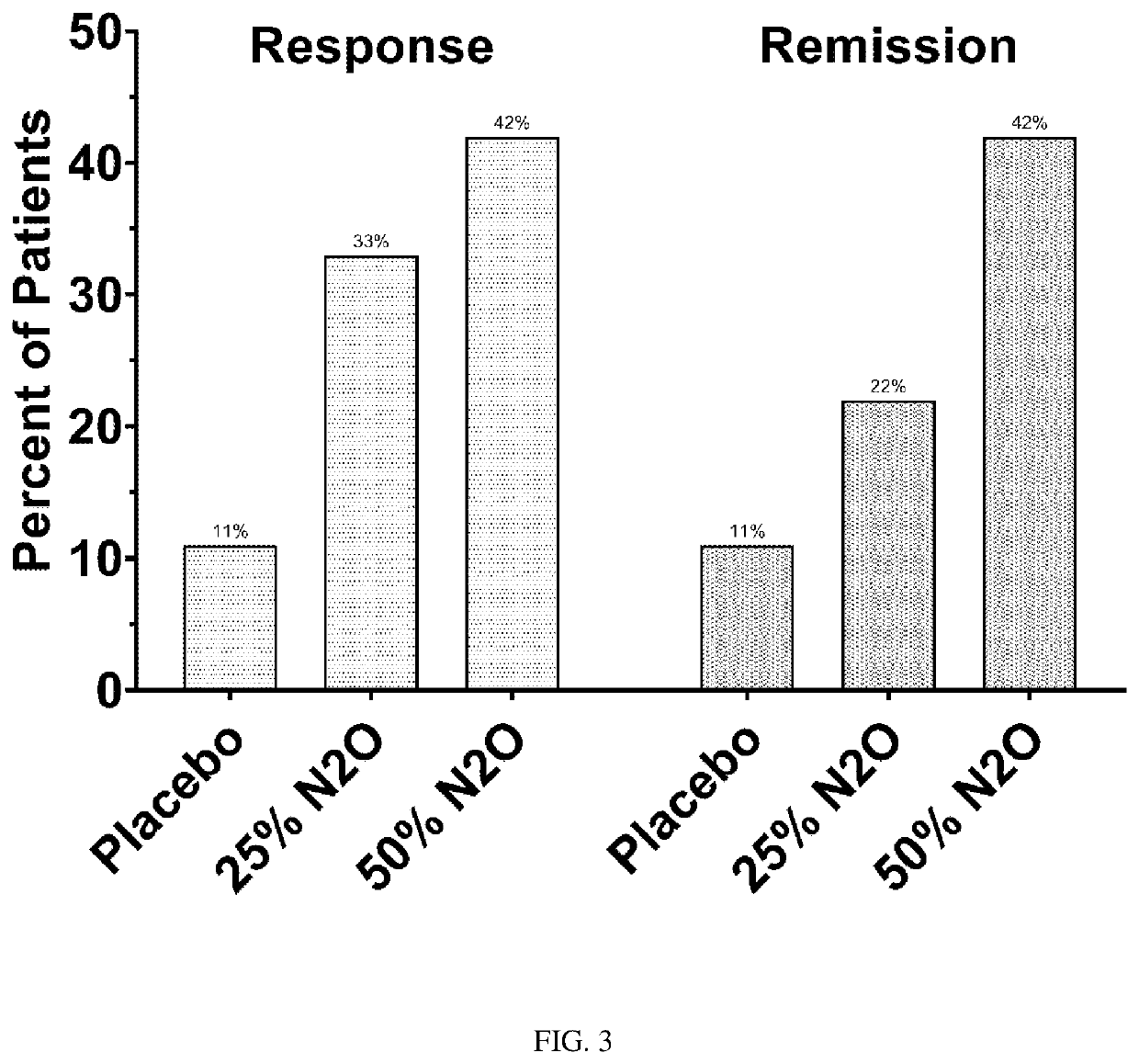
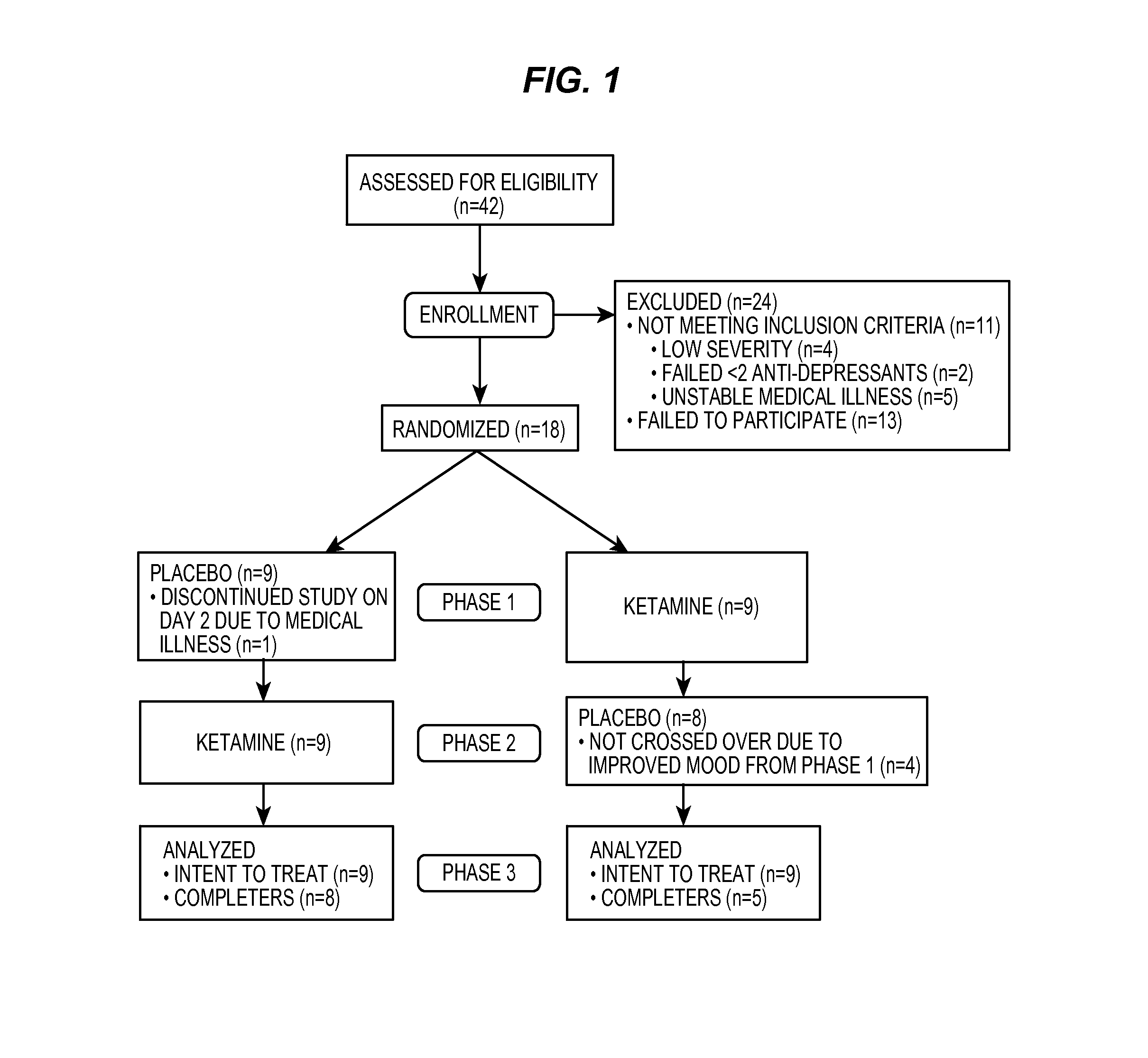
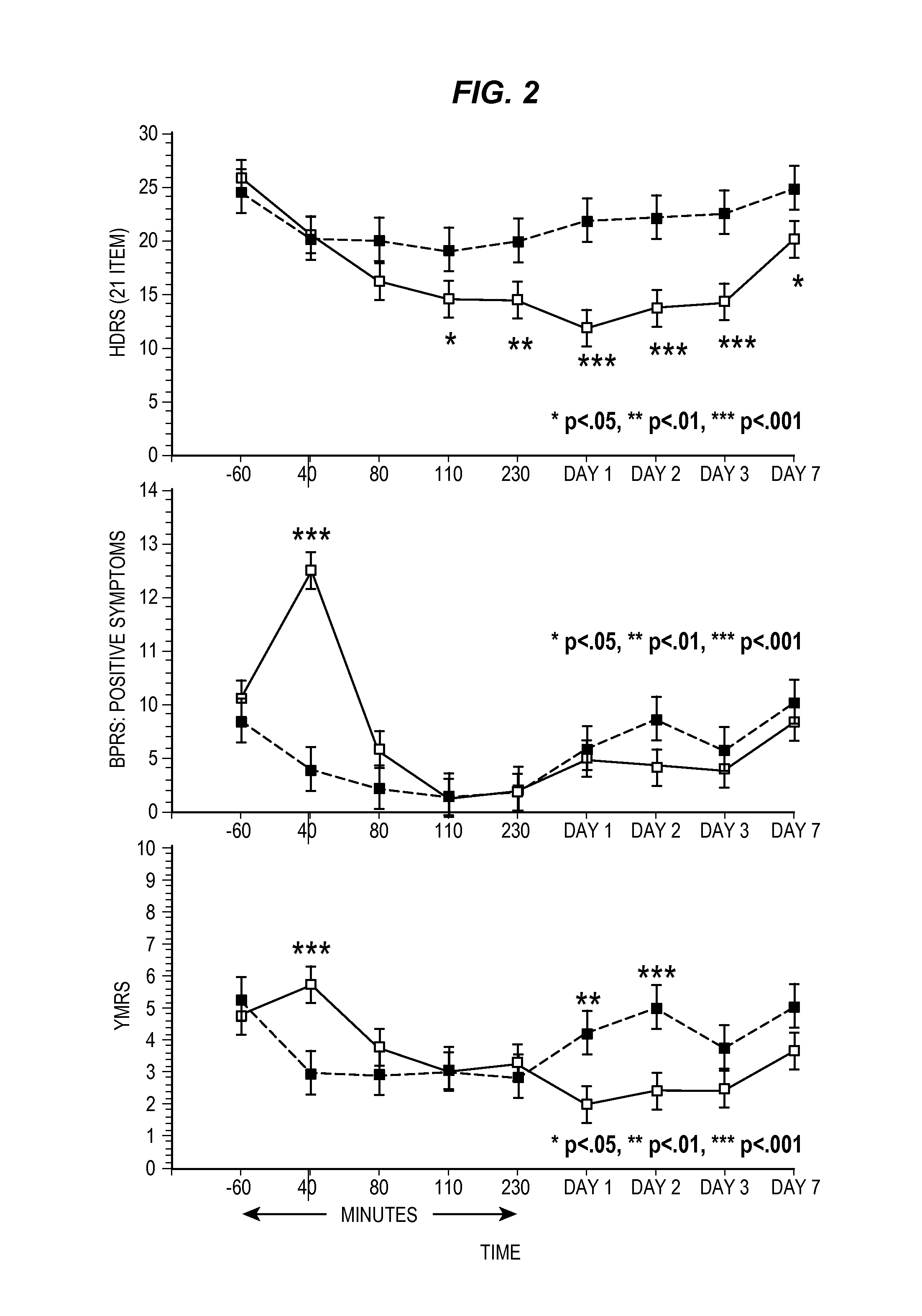
Compositions and methods for treating treatment-resistant depressive disorders with nitrous oxide
PendingUS20220313728A1Similar efficacyEliminate side effectsNervous disorderInorganic active ingredientsNitrous oxidePsychiatry
The present invention generally relates to the use of 25% nitrous oxide for treating patients with a treatment-resistive depressive disorder and compositions useful for the same.
Owner:NAGELE PETER
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com


![4-[2,3-Difluoro-6-(2-fluoro-4-methyl-phenylsulfanyl)-phenyl]-piperidine 4-[2,3-Difluoro-6-(2-fluoro-4-methyl-phenylsulfanyl)-phenyl]-piperidine](https://images-eureka.patsnap.com/patent_img/13264ff2-4046-40b5-8301-1bda1efbba26/US20110039890A1-20110217-C00001.png)
![4-[2,3-Difluoro-6-(2-fluoro-4-methyl-phenylsulfanyl)-phenyl]-piperidine 4-[2,3-Difluoro-6-(2-fluoro-4-methyl-phenylsulfanyl)-phenyl]-piperidine](https://images-eureka.patsnap.com/patent_img/13264ff2-4046-40b5-8301-1bda1efbba26/US20110039890A1-20110217-C00002.png)
![4-[2,3-Difluoro-6-(2-fluoro-4-methyl-phenylsulfanyl)-phenyl]-piperidine 4-[2,3-Difluoro-6-(2-fluoro-4-methyl-phenylsulfanyl)-phenyl]-piperidine](https://images-eureka.patsnap.com/patent_img/13264ff2-4046-40b5-8301-1bda1efbba26/US20110039890A1-20110217-C00003.png)
![Dibenzo[b,f][1,4]oxazapine compounds Dibenzo[b,f][1,4]oxazapine compounds](https://images-eureka.patsnap.com/patent_img/5459d41b-9c82-4a03-a589-e7c2516218b7/US08093237-20120110-D00001.png)
![Dibenzo[b,f][1,4]oxazapine compounds Dibenzo[b,f][1,4]oxazapine compounds](https://images-eureka.patsnap.com/patent_img/5459d41b-9c82-4a03-a589-e7c2516218b7/US08093237-20120110-D00002.png)
![Dibenzo[b,f][1,4]oxazapine compounds Dibenzo[b,f][1,4]oxazapine compounds](https://images-eureka.patsnap.com/patent_img/5459d41b-9c82-4a03-a589-e7c2516218b7/US08093237-20120110-D00003.png)
![DIBENZO[b,f][1,4]OXAZAPINE COMPOUNDS DIBENZO[b,f][1,4]OXAZAPINE COMPOUNDS](https://images-eureka.patsnap.com/patent_img/d45b77f7-a7e9-45bc-a40b-102f6bd1a270/US20080255088A1-20081016-D00000.png)
![DIBENZO[b,f][1,4]OXAZAPINE COMPOUNDS DIBENZO[b,f][1,4]OXAZAPINE COMPOUNDS](https://images-eureka.patsnap.com/patent_img/d45b77f7-a7e9-45bc-a40b-102f6bd1a270/US20080255088A1-20081016-D00001.png)
![DIBENZO[b,f][1,4]OXAZAPINE COMPOUNDS DIBENZO[b,f][1,4]OXAZAPINE COMPOUNDS](https://images-eureka.patsnap.com/patent_img/d45b77f7-a7e9-45bc-a40b-102f6bd1a270/US20080255088A1-20081016-D00002.png)