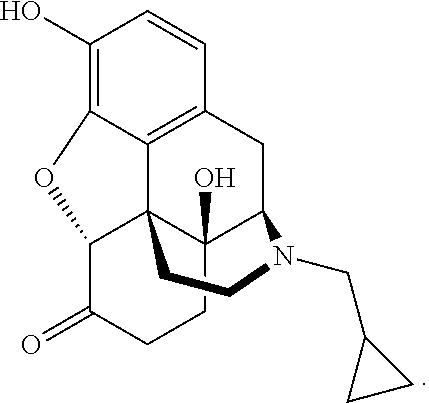
Treatments For Depression And Other Diseases With A Low Dose Agent
a low-dosage agent and treatment technology, applied in the direction of biocide, drug composition, metabolic disorder, etc., can solve the problems of depression symptoms that are difficult to stay focused and paying attention, and depression symptoms that are difficult to cope with
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Patient Responses to Low Dose Naltrexone Treatment of Depression
[0155]Patient #1: A middle aged female suffered severe depression with recurrent major depressive episodes. She had responded briefly to complex polypharmacy and electroconvulsive therapy, but depression usually returned within 3 weeks of these interventions. In addition to restarting aripiprazole (ABILIFY), to which she had transiently responded in the past, the patient was instructed to pulverize a tablet of naltrexone 50 mg, and take the smallest fragment with water. A sample of these fragments was later weighed, indicating she was taking on the average about 1 mg of naltrexone daily. The former pharmacologic regimen was otherwise unchanged.
[0156]About one week after initiating low dose naltrexone, the patient experienced marked remission. Despite minor variation in mood, she has maintained the remission for more than 6 months (continuing to present). She has remained on adjunctive naltrexone 1 mg daily.
[0157]Patient...
example 2
Clinical Testing of Low Dose Naltrexone Treatment of Depression
[0165]The clinical studies described herein, in part, assess the magnitude and rate of response to low dose naltrexone, as measured by change on the HAM-D-17, compared to placebo. Low dose naltrexone possesses a unique antidepressant signature distinct from the more gradual rates of symptom change characteristic of both placebo response and response to first-line antidepressant agents.
[0166]The trial is conducted over 6 weeks, with double-blind treatment divided into two phases of 3 weeks each; this interval of 3 weeks was chosen based on the rapid course of recovery described in the case reports above. The first phase consists of a 1:1 randomization between placebo (n=18) and active treatment (n=18). Entering phase 2, all placebo non-responders are switched to low dose naltrexone.
[0167]12 men and women ages 18 to 65 who have received adequately dosed SSRI (optionally in combination with a dopaminergic agent), or a selec...
PUM
| Property | Measurement | Unit |
|---|---|---|
| length | aaaaa | aaaaa |
| physical health | aaaaa | aaaaa |
| concentration | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com