Combined medicine for curing treatment-resistant depression and applications thereof
A depression and refractory technology, applied in drug combinations, medical preparations containing active ingredients, pharmaceutical formulas, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0015] (1) Method
[0016] 1.1 Object
[0017] This study is a randomized, double-blind multi-center (8 centers) study, from January 2005 to December 2007, inclusion and exclusion criteria:
[0018] (1) Meet the diagnostic criteria of DSM-IV "major depressive episode";
[0019] (2) The current episode has been treated for 2 months, and the 17-item Hamilton Depression Scale (HAMD-17) score is still ≥ 17 points;
[0020] (3) The 17-item Hamilton Depression Scale (HAMD-17) score was still ≥ 17 at the time of enrollment;
[0021] (4) 18-65 years old, male or female;
[0022] (5) Junior high school education or above;
[0023] (6) Sufficient audio-visual level to complete the examinations necessary for the study.
[0024] (7) Exclude those who are currently suffering from serious physical diseases, those who have serious suicide attempts, and pregnant or lactating women;
[0025] (8) Exclude patients diagnosed with bipolar disorder who are currently suffering from depression;...
Embodiment 2
[0054] Chinese patent application number 200910002812.9 discloses an antidepressant drug combination preparation and its application method and use. The invention discloses an antidepressant drug combination preparation, especially a drug combination preparation comprising trazodone and other antidepressant drugs. , and its use in the preparation of medicaments for treating depression.
[0055] Fluoxetine + trazodone group: prepare the joint preparation according to the method disclosed in Chinese Patent Application No. 200910002812.9, fluoxetine (44.8 mg) + trazodone (100 mg);
[0056] Paroxetine + trazodone group: paroxetine (20mg) + trazodone (100mg);
[0057] Paroxetine + sodium valproate group: paroxetine (20mg) + sodium valproate (600mg).
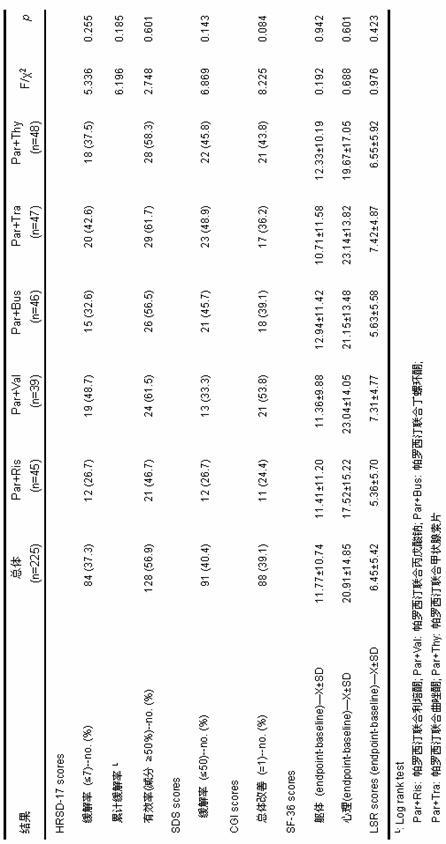
[0058] Referring to the method of Example 1, 150 patients with refractory depression were randomly divided into 3 groups, and received the above three combination drugs for 8 weeks. A total of 121 (80.7%) patients completed the 8-we...
Embodiment 3
[0061] The preparation of embodiment 3 combination pharmaceutical preparations
[0062] Recipe 1:
[0063] Name of raw material
[0064] Recipe 2:
[0065] Name of raw material
[0066] Carboxymethyl Starch Sodium
[0067] Recipe 3:
[0068] Name of raw material
[0069] Recipe 4:
[0070] Name of raw material
[0071] Recipe 5:
[0072] Name of raw material
[0073] Preparation process: pass through 80-mesh sieve for paroxetine hydrochloride, 100-mesh sieve for sodium valproate, 80-mesh sieve for lactose and carboxymethyl starch sodium, 100-mesh sieve for starch, and 40-mesh sieve for magnesium stearate. Mix paroxetine hydrochloride and lactose evenly, then mix evenly with sodium valproate, then mix evenly with starch, granulate with starch slurry, dry in a 50-degree oven, granulate with a 20-mesh sieve, and punch out φ8mm tablets.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com