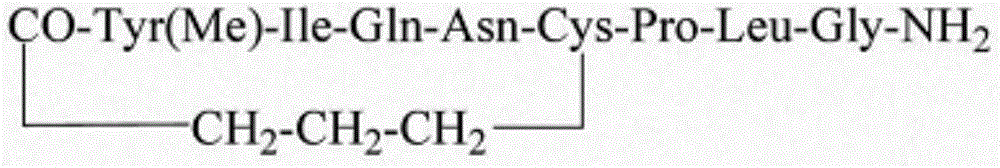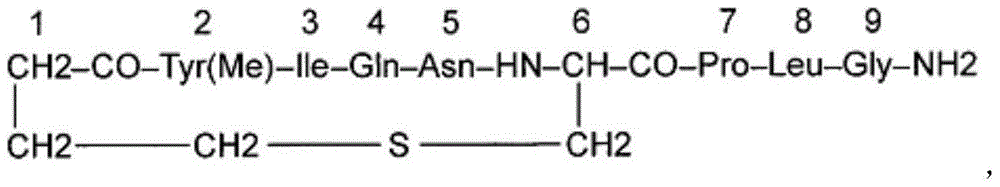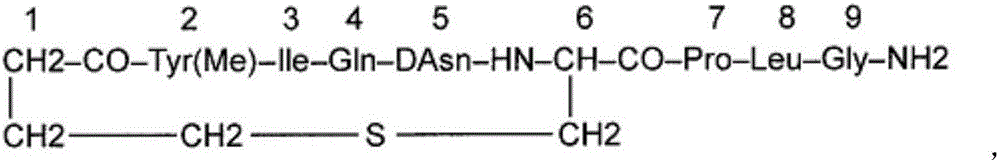Carbetocin injection and its preparation method
A technology of carbetocin and injection, applied in the field of medicine, can solve the problems of carbetocin chemical sensitivity, strict quality requirements, impurity control requirements, high content control requirements, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0211] Embodiment 1: Preparation of carbetocin injection
[0212] formula:
[0213] Carbetocin
0.1 mg,
Dextran 40
10mg,
pH regulator
Appropriate amount, adjust the pH value to 4.0,
Water for Injection
Appropriate amount, add to 1ml.
[0214] Preparation method:
[0215] (1) Dissolve each solid excipient and carbetocin in an appropriate amount of water for injection, so that the volume of the solution reaches 80% of the theoretical prescription volume (ie about 0.8ml, the same below);
[0216] (2) Regulate the pH value of the solution to a specified value with an acid-base regulator;
[0217] (3) Add water for injection to 1ml, add 0.05% activated carbon to the liquid medicine, absorb pyrogen for 30 minutes under stirring, decarburize and filter. The filtrate was filtered through a 0.22 μm titanium rod filter, and then sterilized and filtered through a 0.22 μm microporous membrane, and 1 ml of the liquid medicine was fille...
Embodiment 2
[0235] Embodiment 2: Preparation of carbetocin injection
[0236] formula:
[0237] Carbetocin
0.1 mg,
Dextran 40
10mg,
2.5 mg,
pH regulator
Appropriate amount, adjust the pH value to 4.0,
Water for Injection
Appropriate amount, add to 1ml.
[0238] Preparation method:
[0239] (1) Dissolve each solid excipient and carbetocin in an appropriate amount of water for injection, so that the volume of the solution reaches 80% of the theoretical prescription volume (ie about 0.8ml, the same below);
[0240] (2) Regulate the pH value of the solution to a specified value with an acid-base regulator;
[0241] (3) Add water for injection to 1ml, add 0.05% activated carbon to the liquid medicine, absorb pyrogen for 30 minutes under stirring, decarburize and filter. The filtrate was filtered through a 0.22 μm titanium rod filter, and then sterilized and filtered through a 0.22 μm microporous membrane, and 1 ml ...
Embodiment 3
[0251] Embodiment 3: Preparation of carbetocin injection
[0252] formula:
[0253] Carbetocin 2
[0254] Chlorobutanol
[0255] Prepared with reference to the method of Example 1, the injection Ex3 was obtained.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com