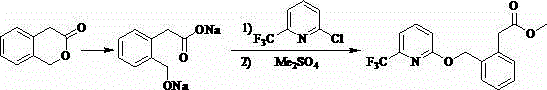Preparation method of agricultural bactericide
A technology of agricultural fungicides and inert solvents, applied in the field of preparation of agricultural fungicides
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
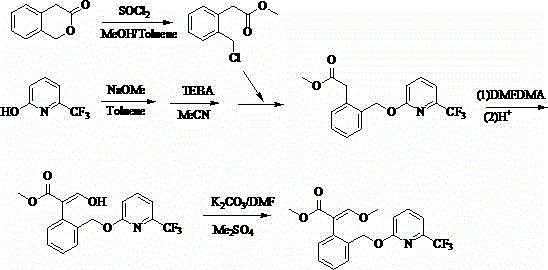
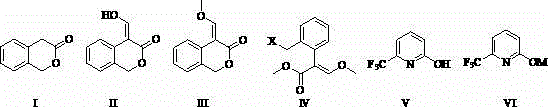
[0041] The preparation method of a kind of agricultural fungicide of the present embodiment, as shown in table 1, comprises the following steps: 1) 3-isochromanone (I) 40 g (0.27 mol), add 150 1 mL of dimethylformamide dimethyl acetal (DMFDMA), heated to 40°C to reflux for 4 h, the analysis showed that the reaction had stopped, the solvent was distilled off under reduced pressure, and 200 mL of toluene and 21.4 g (0.09 mo1) of chlorine hexahydrate were added Nickel, stirred at room temperature for 3h, filtered, and the solid was washed with 30mL of toluene. The filtrate was concentrated to obtain 4-(α-hydroxy)methylene-2-H-chromen-3-(4 H)-one (II), which could be directly used in the next reaction.
[0042] 2) Dissolve 4-(α-hydroxy)methylene-2-H-benzopyran-3-(4 H)-one (II) obtained in step 1) in 150 mL dimethyl sulfoxide, and stir Add 41.4 g (0.3 mol) of potassium carbonate and 50.4 g (0.4 mol) of dimethyl sulfate to it, and control the temperature at 40 ° C. After reacting f...
Embodiment 2
[0046] The preparation method of a kind of agricultural fungicide of the present embodiment, the step is roughly as embodiment 1, and its difference is: described step 2) in the molar ratio of alkali and 3-isochromanone (I) is 1:1; the molar ratio of methylating reagent to 3-isochromanone (I) is 1.5:1; halogenating reagent and 4-(methoxymethenyl)-3-isobenzo The molar ratio of pyrone (III) is 1.5:1; the molar ratio of base and 2-hydroxy-6-trifluoromethylpyridine (V) in the step 4) is 1.1:1; other differences are shown in the table shown in 1. Crude product obtains 74.2g picoxystrobin through crystallization, mother liquor concentrates and continues crystallization to obtain 9g picoxystrobin, adds up to 83.2g, content 98.4% (external standard method detects), yield 82% (with starting material 3-isobenzo Dihydropyrone (I) 40g is a benchmark). This embodiment has a short reaction route, simple and safe operation, mild conditions, simple and easy post-treatment, less waste, hig...
Embodiment 3
[0048] The preparation method of a kind of agricultural fungicide of the present embodiment, the step is roughly as embodiment 1, and its difference is: described step 2) in the molar ratio of alkali and 3-isochromanone (I) is 1:1; the molar ratio of methylating reagent to 3-isochromanone (I) is 1.5:1; halogenating reagent and 4-(methoxymethenyl)-3-isobenzo The molar ratio of pyrone (III) is 1.5:1; the molar ratio of base and 2-hydroxyl-6-trifluoromethylpyridine (V) in the step 4) is 1.2:1; other differences are shown in the table shown in 1. Crude product obtains 76g picoxystrobin through crystallization, mother liquor concentrates and continues to crystallize and obtains 9.6g picoxystrobin, adds up to 85.6g, content 98.2% (external standard method detects), yield 84.8% (with starting material 3-isobenzo Dihydropyrone (I) 40g is a benchmark). This embodiment has a short reaction route, simple and safe operation, mild conditions, simple and easy post-treatment, less waste, h...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com