A kind of semiconducting conjugated polymer and its synthesis method
A technology of conjugated polymers and semiconductors, applied in the direction of organic chemistry, can solve the problems of low energy level, unfavorable electron injection and stable transport, and less electron transport materials, and achieve the effect of low unoccupied orbital energy level
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0027] The preparation method of each monomer is described as follows:
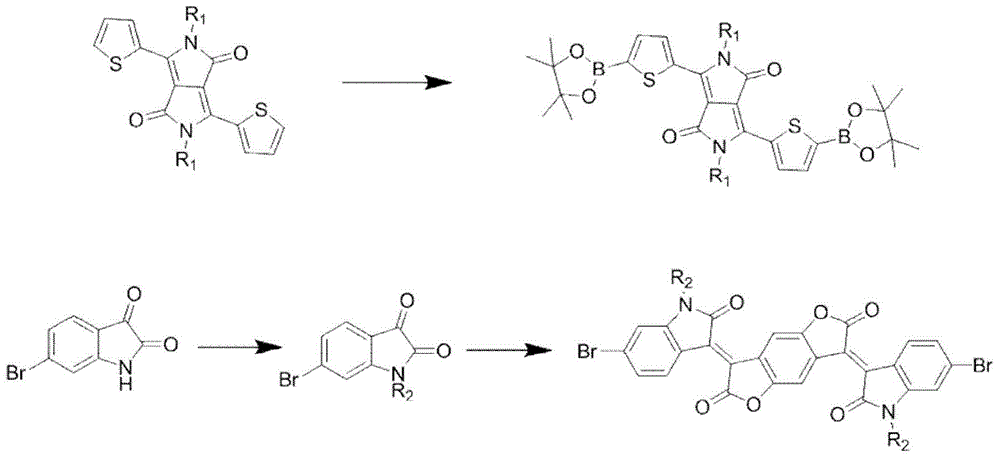
[0028] Preparation of (thienyl)pyrrolopyrrole-diketopinacol diboron monomer
[0029] (Thienyl) pyrrolopyrrole-diketone pinacol diboron monomer synthetic route schematic diagram as figure 2 As shown, the detailed preparation method can be found in literature reports (Advance Materials, 2013, 25, 5467-5472.).
[0030] Preparation of (2-oxindol-3-ylidene)benzodifuran-dione dibromomonomer
[0031] (2-oxindol-3-ylidene) benzodifuran-diketone dibromomonomer synthetic route such as figure 2 shown. For the detailed preparation method, please refer to the literature report (Chemical Communication, 2013, 49, 3790-3792.).
Embodiment 1
[0032] Embodiment 1, synthetic polymer P 1
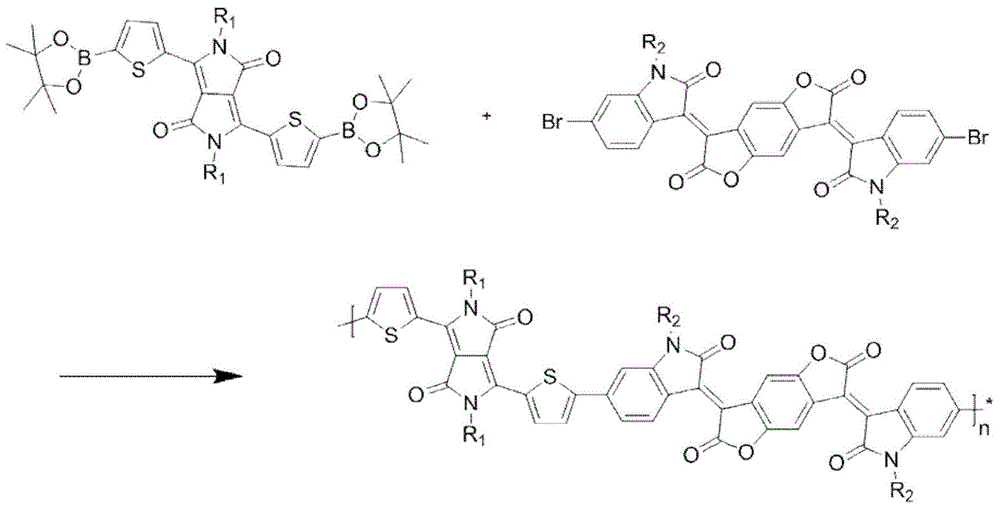
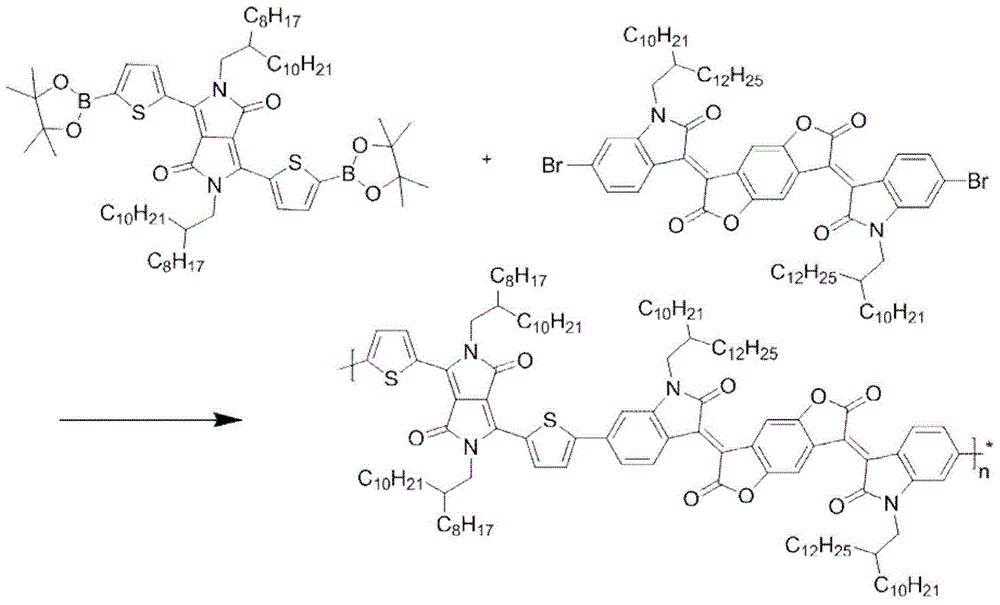
[0033] Polymer P 1 The synthetic route of image 3 Shown, concrete steps are: in 100mL reaction bottle, add 0.5mmol bistin monomer and 0.5mmol bibromine monomer (R 1 and R 2 As shown in Table 1), then add 10mL toluene, replace with nitrogen for 40 minutes, add 2.5mmol of K 3 PO 4 2 mL of aqueous solution, 2% catalyst tris(dibenzylideneacetone) dipalladium, and tris(o-methylphenyl)phosphorus as a ligand, reacted at 90°C for 24 hours, cooled the reaction to room temperature, added 200 mL of methanol for precipitation, The solid was filtered, extracted by Soxhlet with methanol and n-hexane for 24 hours, and then extracted by Soxhlet with chloroform for 24 hours. Finally, the liquid was rotary evaporated, and methanol precipitated to obtain a black polymer.
Embodiment 2-3
[0035] The specific steps are the same as in Example 1: add 0.5 mmol of bistin monomer and 0.5 mmol of bis-bromine monomer (R1 and R2 are shown in Table 1) in a 100 mL reaction flask, add 10 mL of toluene, replace with nitrogen for 40 minutes, and then add 2.5 mmol K 3 PO 4 2 mL of aqueous solution, 2% catalyst tris(dibenzylideneacetone) dipalladium, and tris(o-methylphenyl)phosphorus as ligand, reacted at 90°C for 24 hours, cooled to room temperature, added 200 mL of methanol to precipitate, solid Soxhlet extraction with methanol and n-hexane for 24 hours, and then Soxhlet extraction with chloroform for 24 hours, and finally the liquid was rotary evaporated, and methanol precipitated to obtain black polymers P2-P3, the specific structure of which is shown in Table 1.
[0036] Figure 4 The absorption spectrum of polymer P1 is given, and the absorption peak covers visible light and extends to the near-infrared region.
[0037]In summary, the novel semiconductor conjugated p...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com