Improved method for preparing 4-cyanobiphenyl
A technology for biphenyl and biphenylcarboxamide, which is applied in the field of preparing 4-biphenyl nitrile, can solve the problems of expensive catalyst, harsh reaction conditions, low total yield, etc., and achieves the effects of easy control of the reaction process and reduction of operating costs.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
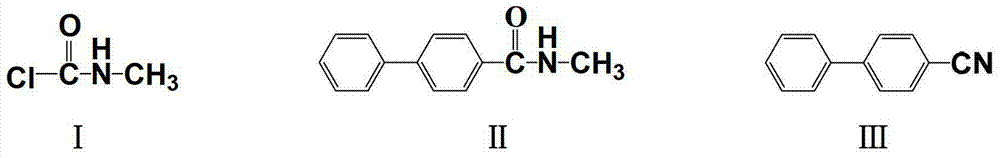
[0027] Add dichloromethane (150g) and anhydrous aluminum trichloride (19.1g, 0.145mol) into the reaction flask, stir for 1 hour, so that anhydrous aluminum trichloride is uniformly dispersed in the solvent, add biphenyl (14.6g, 85.7mmol), after it dissolves, slowly add N-methylcarbamoyl chloride (9.67g, 103mmol) dropwise. After the gas no longer escapes, remove the ice-water bath, wait for the temperature to return to ambient temperature, then heat to 40°C, react for 5 hours, stop the reaction, slowly pour the reactant into ice water with pH=3, separate layers, organic The phase was washed with water until neutral, the solvent was evaporated to dryness under reduced pressure, and the residue was recrystallized from ethanol to obtain 15.5 g of N-methyl-4-biphenylcarboxamide with a purity of 98.9% (HPLC) and a yield of 79.6%.
[0028] Add N-methyl-4-biphenylcarboxamide (10g, 47mmol) and 1,2,4-trichlorobenzene (250g) into the reaction flask, and heat until N-methyl-4-biphenylcarb...
Embodiment 2
[0030] Add dichloroethane (150g) and anhydrous aluminum trichloride (25g, 189mmol) into the reaction bottle, stir for 1 hour, so that anhydrous aluminum trichloride is evenly dispersed in the solvent, add biphenyl (14.6g, 94.5 mmol), after it dissolves, slowly add N-methylcarbamoyl chloride (9.67g, 104mmol) dropwise. After the gas no longer escapes, remove the ice-water bath, wait for the temperature to return to ambient temperature, then heat to 40°C, react for 5 hours, stop the reaction, slowly pour the reactant into ice water with pH=3, separate layers, organic The phase was washed with water until neutral, the solvent was evaporated to dryness under reduced pressure, and the residue was recrystallized from ethanol to obtain 15.95 g of N-methyl-4-biphenylcarboxamide with a purity of 98.4% (HPLC) and a yield of 92.8%.
[0031]Add N-methyl-4-biphenylcarboxamide (10g, 47mmol) and o-dichlorobenzene (250g) into the reaction flask, heat until N-methyl-4-biphenylcarboxamide is com...
Embodiment 3
[0033] Add dichloroethane (1000g) and anhydrous aluminum trichloride (252g, 1.89mol) into the reaction bottle, stir for 1 hour, so that anhydrous aluminum trichloride is evenly dispersed in the solvent, add biphenyl (146g, 0.945 mol), after it dissolves, slowly add N-methylcarbamoyl chloride (103g, 1.10mol) dropwise. After the gas no longer escapes, remove the ice-water bath, wait for the temperature to return to ambient temperature, then heat to 40°C, react for 5 hours, stop the reaction, slowly pour the reactant into ice water with pH=3, separate layers, organic The phase was washed with water to neutrality, the solvent was evaporated to dryness under reduced pressure, and the residue was recrystallized from ethanol to obtain 160 g of N-methyl-4-biphenylcarboxamide with a purity of 98.4% (HPLC) and a yield of 74.5%.
[0034] Add N-methyl-4-biphenylcarboxamide (106.8g, 470mmol) and o-dichlorobenzene (2000g) into the reaction flask, heat until N-methyl-4-biphenylcarboxamide is...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com