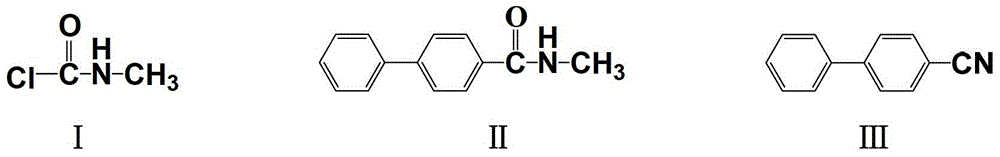
A kind of improved method of preparing 4-biphenylnitrile
A technology of biphenyl and biphenylcarboxamide, which is applied in the field of preparing 4-biphenylnitrile, can solve the problems of expensive catalyst, harsh reaction conditions, and low overall yield, and achieve the effect of easy control of the reaction process and reduced operating costs
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0027] Dichloromethane (150g) and anhydrous aluminum trichloride (19.1g, 0.145mol) were added to the reaction flask, and stirred for 1 hour to make the anhydrous aluminum trichloride evenly dispersed in the solvent, and biphenyl (14.6g, 0.145mol) was added. 85.7 mmol), after it was dissolved, N-methylcarbamoyl chloride (9.67 g, 103 mmol) was slowly added dropwise. During this period, the temperature of the reactant was kept at 0-5 °C with an ice-water bath. At this time, a large amount of gas escaped. After the gas no longer escapes, remove the ice-water bath, and after the temperature rises back to the ambient temperature, then heat to 40 °C, react for 5 hours, stop the reaction, slowly pour the reactant into ice water with pH=3, layer, and organically The phase was washed with water until neutral, the solvent was evaporated to dryness under reduced pressure, and the residue was recrystallized from ethanol to obtain 15.5 g of N-methyl-4-biphenylcarboxamide with a purity of 98....
Embodiment 2
[0030] Dichloroethane (150g) and anhydrous aluminum trichloride (25g, 189mmol) were added to the reaction flask, and stirred for 1 hour to make the anhydrous aluminum trichloride evenly dispersed in the solvent, and biphenyl (14.6g, 94.5g) was added. mmol), after it was dissolved, N-methylcarbamoyl chloride (9.67g, 104mmol) was slowly added dropwise. During this period, the temperature of the reactant should be kept at 0-5°C with an ice-water bath. At this time, a large amount of gas escaped. After the gas no longer escapes, remove the ice-water bath, and after the temperature rises back to the ambient temperature, then heat to 40 °C, react for 5 hours, stop the reaction, slowly pour the reactant into ice water with pH=3, layer, and organically The phase was washed with water until neutral, the solvent was evaporated to dryness under reduced pressure, and the residue was recrystallized from ethanol to obtain 15.95 g of N-methyl-4-biphenylcarboxamide with a purity of 98.4% (HPLC...
Embodiment 3
[0033] Dichloroethane (1000g) and anhydrous aluminum trichloride (252g, 1.89mol) were added to the reaction flask, and stirred for 1 hour to make the anhydrous aluminum trichloride evenly dispersed in the solvent, and biphenyl (146g, 0.945 g) was added. mol), after it dissolves, slowly add N-methylcarbamoyl chloride (103 g, 1.10 mol) dropwise. During this period, keep the temperature of the reactant at 0-5 °C with an ice-water bath. At this time, a large amount of gas escapes. After the gas no longer escapes, remove the ice-water bath, and after the temperature rises back to the ambient temperature, then heat to 40 °C, react for 5 hours, stop the reaction, slowly pour the reactant into ice water with pH=3, layer, and organically The phase was washed with water until neutral, the solvent was evaporated to dryness under reduced pressure, and the residue was recrystallized from ethanol to obtain 160 g of N-methyl-4-biphenylcarboxamide with a purity of 98.4% (HPLC) and a yield of 7...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com