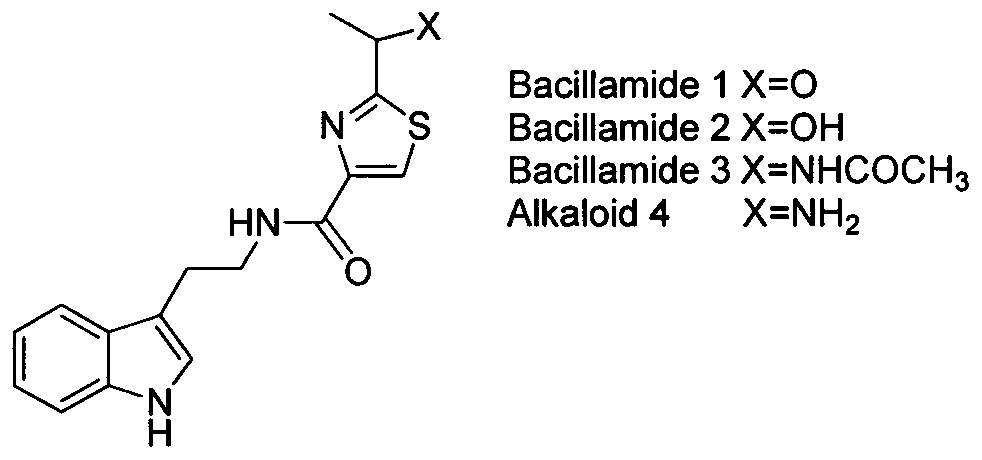
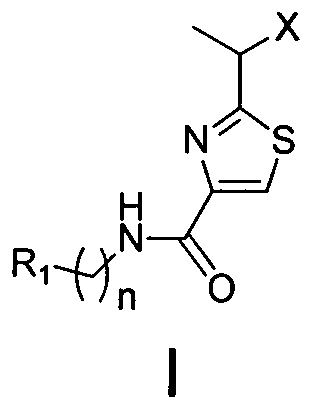
2-(1-ethoxyl).2-acetylthiazole-4-formamide compounds and application
A technology of acetylthiazole and formamide, which is applied in application, organic chemistry, biocide, etc., can solve the problems of few compounds, no specific research on the biological activity of freshwater algae, lack of test receptors, etc., and achieve good stability , low price, significant social and economic benefits
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
[0018] The present invention is further described below in conjunction with embodiment, and its purpose is that content of the present invention can be better understood and embodies substantive characteristics of the present invention, so the example given should not be considered as limiting the protection scope of the present invention.
[0019] For the experimental methods that do not indicate specific conditions in the examples, usually follow the conventional conditions and the conditions described in the manual, or according to the conditions suggested by the manufacturer; used equipment, materials, reagents, etc., if no special instructions, can be obtained from obtained commercially.
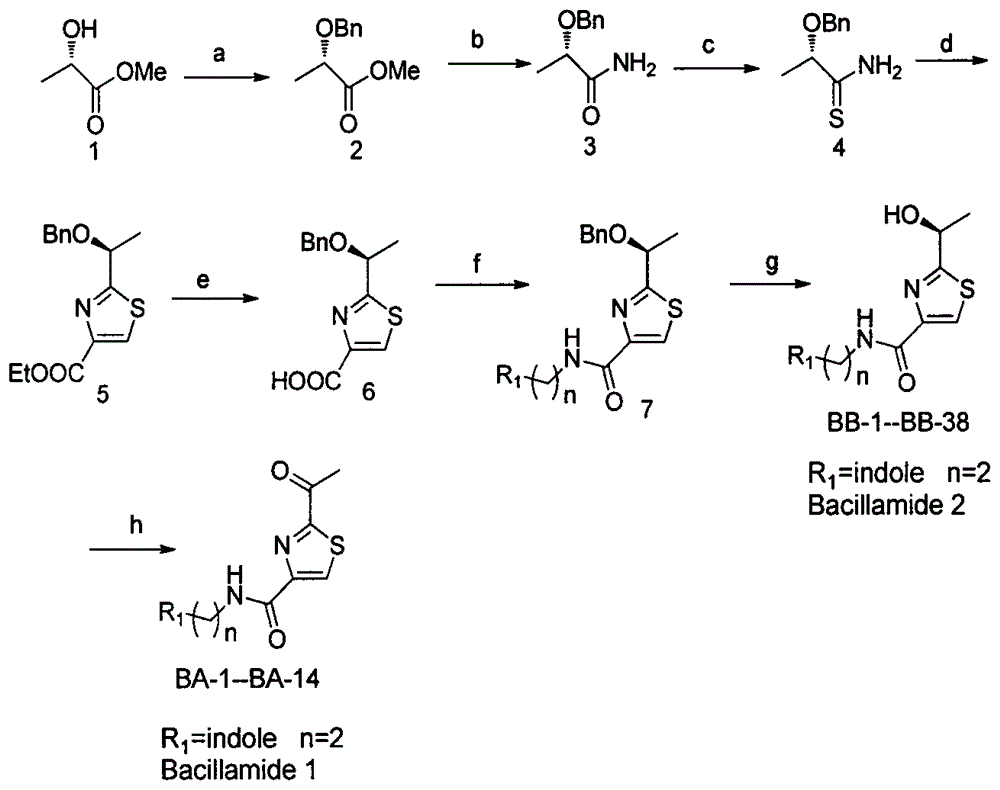
[0020] 1) Synthesis of benzyl-protected L-lactate methyl ester 2
[0021] Dissolve L-methyl lactate (1.04g, 0.01mol) in 20ml of THF at -20°C, then slowly add 60% sodium hydride (0.508g, 0.0127mol), stir for 20min, then add benzyl bromide (2.48 g, 0.0145mol), after the addition was comp...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com