Hydroxamic acid micromolecule organic compound with thaizolidinone structure as well as derivatives, application and preparation method of hydroxamic acid micromolecule organic compounds
A technology of organic compounds and thiazolinones, applied in the field of medicine, can solve the problems of loss of activity of tumor suppressor genes and high activation of proto-oncogenes
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0061]
Embodiment 1-1
[0062] Example 1-1, Preparation of Compound 4-{2-[2-(3-bromophenyl)-4-carbonyl-3-thiazolyl]phenoxy}-N-hydroxybutyramide (FF001)
[0063] Take 2-aminophenol (1.90g, 10mmol) in toluene (30ml), cool the reaction system with an ice-water bath, add m-bromobenzaldehyde (1.16ml, 10mmol), react at 0°C for 20min, then heat the oil bath to 140°C The water was separated at reflux for 5h, and another reactant, thioglycolic acid (1.39ml, 20mmol), was added. Continue to reflux at 140°C to separate water for 5 hours, remove most of the solvent under reduced pressure, pass through a silica gel column after conventional treatment, and obtain compound I (2.59 g, 74%).
[0064] Compound I (400mg, 1.14mmol) was dissolved in N,N-dimethylformamide (8ml), potassium carbonate (787mg, 2.28mmol) and ethyl bromobutyrate (412mg, 2.28mmol) were added, heated to 50 After reacting at ℃ for about 3 hours, Intermediate II (466 mg, 91%) was isolated.
[0065] Add KOH (2.89g, 51.5mmol) to a solution of hydrox...
Embodiment 2
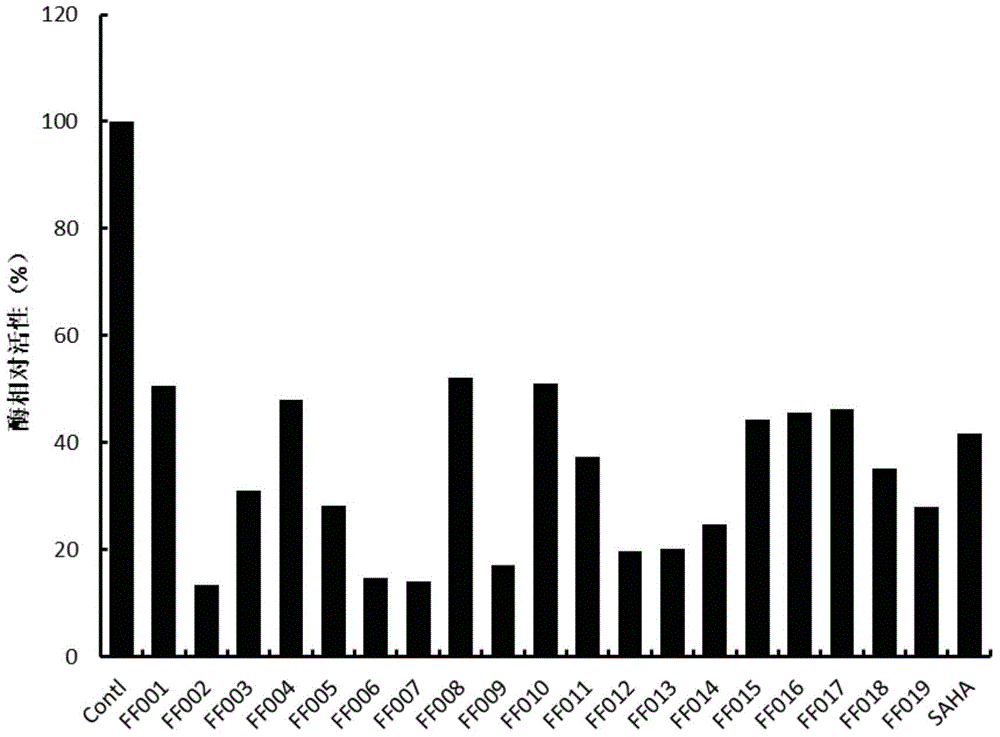
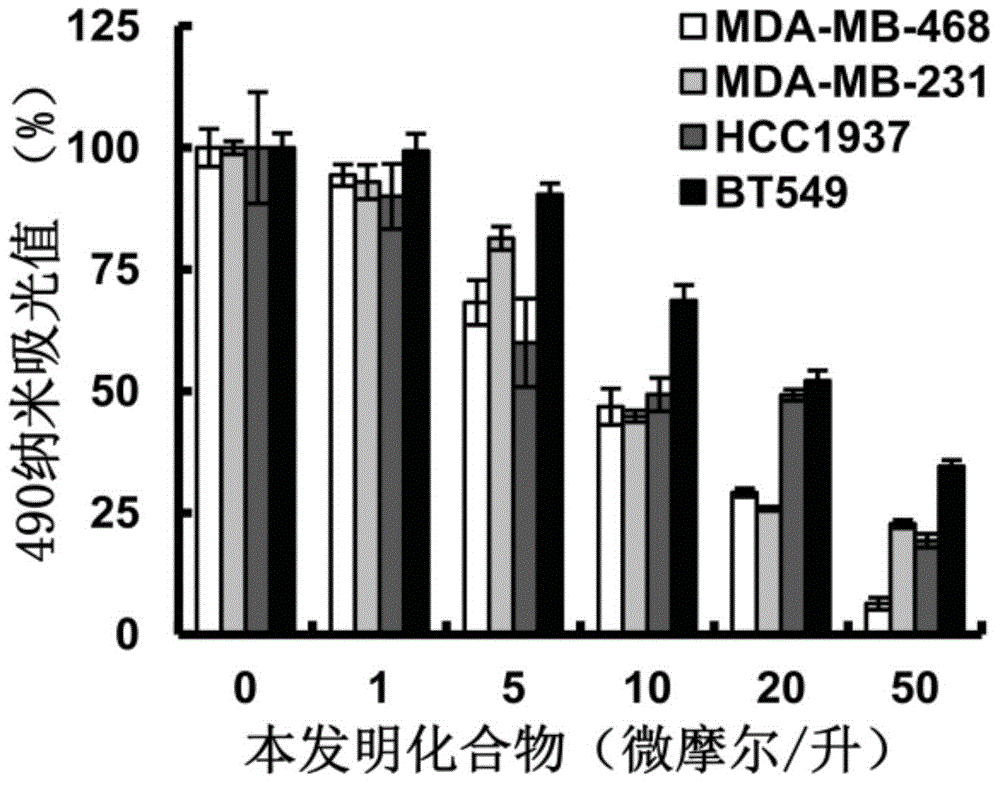
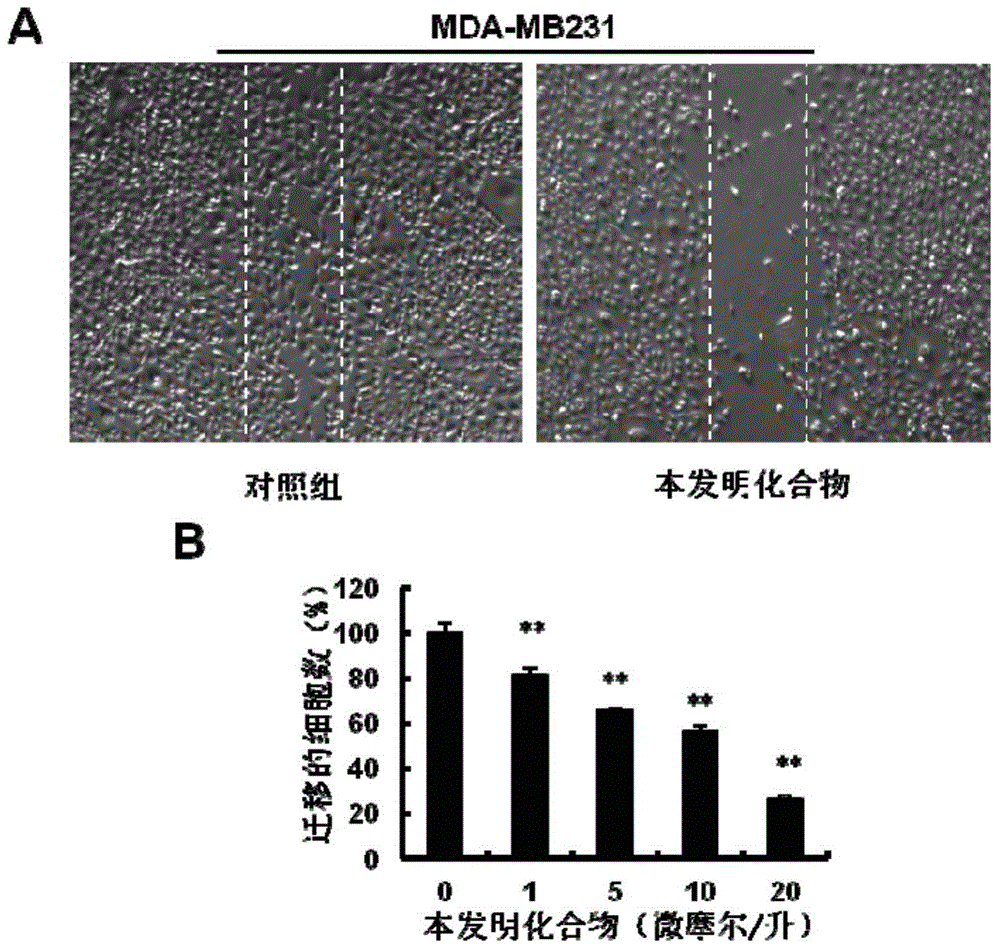
[0071] Example 2. Inhibitory effect of compounds of the present invention on HDACs enzyme activity at the molecular level.
[0072] We first screened the designed and synthesized compounds with novel structures for HDACs enzyme activity using the HDAC enzyme activity screening kit. During the experiment, the compound to be identified was incubated with HDAC active (HeLa or MDA-MB231 cell lysate) and HDAC colorimetric substrate (containing an acetylated lysine side chain. If the substrate is deacetylated, it will be After activation, a luminescent group is generated under the action of lysine chromogen. The final luminescent group is read and analyzed by a microplate reader.
[0073] 1. Add 2 μL of the compound into 85 μL of double-distilled water (add 2 μL of double-distilled water to the control group).
[0074] 2. Add 10 μL of 10× buffer and 2 μL of HeLa nuclear aspirated body fluid to each well.
[0075] 3. Add 5 μL of HDAC fluorescent substrate and incubate in a 37°C inc...
PUM
 Login to view more
Login to view more Abstract
Description
Claims
Application Information
 Login to view more
Login to view more - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap