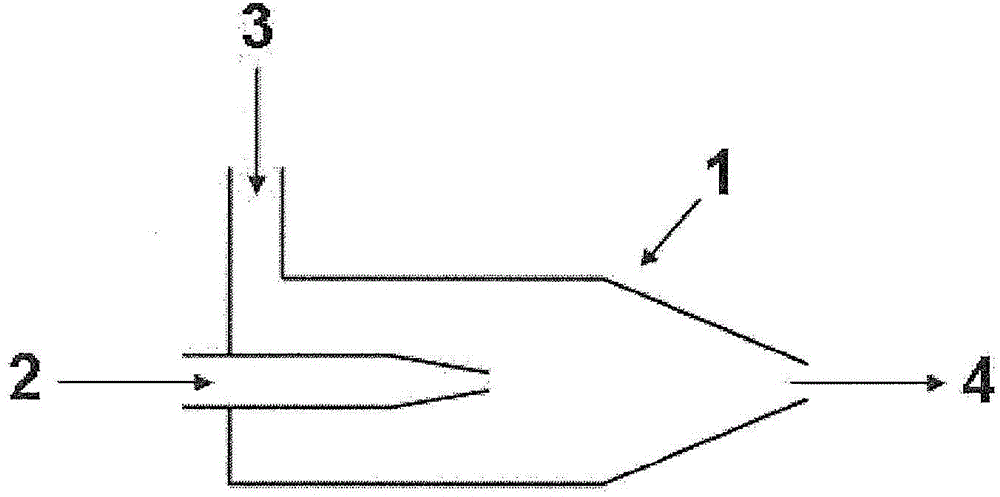
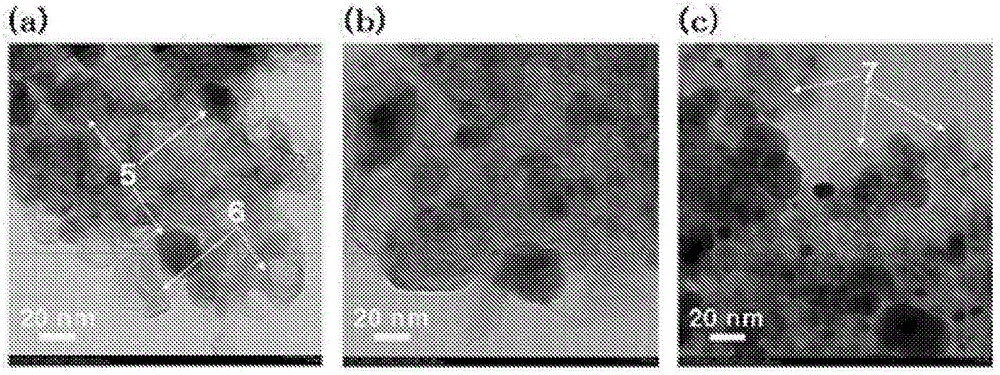
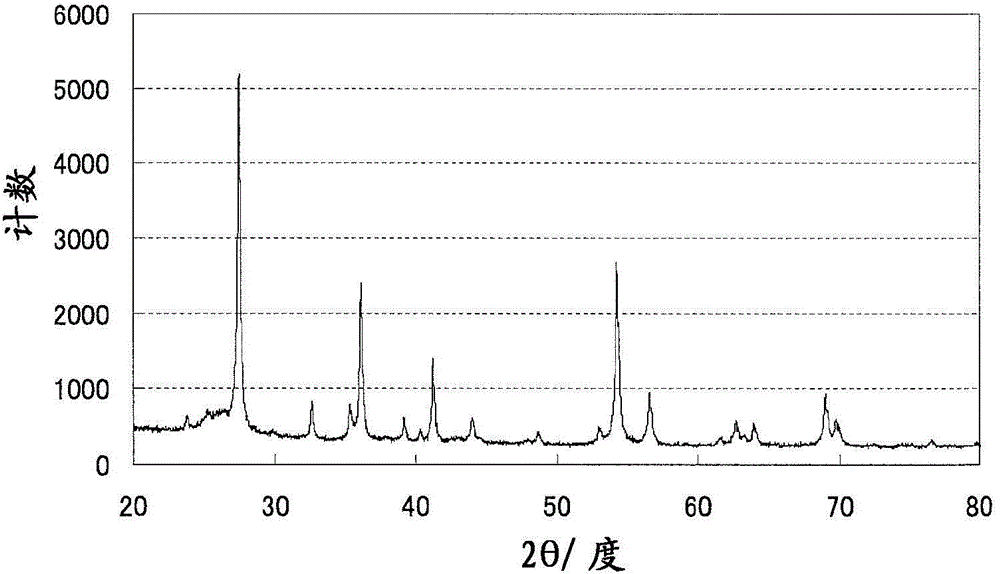
Method for operating fuel cell, and electric-power generating device
A fuel cell and operating method technology, applied to fuel cell parts, fuel cells, fuel cell additives, etc., capable of solving problems such as fuel cell power generation performance degradation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
manufacture example 1
[0214] Operation 1-1
[0215] 5 mL of titanium tetraisopropoxide and 5 mL of acetylacetone were added to a solution of 15 mL of ethanol and 5 mL of acetic acid, and stirred at room temperature to prepare a titanium-containing mixture solution. Separately, 3.76 g of glycine and 0.31 g of iron(II) acetate were added to 20 mL of pure water, and stirred at room temperature to dissolve completely, thereby preparing a glycine-containing mixture solution. Slowly add the titanium-containing mixture solution into the glycine-containing mixture solution to obtain a transparent catalyst precursor solution. Using a rotary evaporator, the temperature of the water bath was set at about 80° C., and the solvent was slowly evaporated while heating and stirring the catalyst precursor solution. The solid residue obtained by completely evaporating the solvent was pulverized finely and uniformly with a mortar to obtain a powder (1a).
[0216] This powder (1a) was placed in a tubular furnace, a...
manufacture example 2、3
[0223] Except changing the amount of distilled water, catalyzer (1), sodium carbonate, chloroplatinic acid hexahydrate and 37% formaldehyde solution as shown in table 1, carried out the operation identical with manufacture example 1, obtains platinum content as table 1 Such different composite catalysts (2), (3).
[0224] In Production Examples 4 to 22, the operations described below and in Table 2 were performed to obtain composite catalysts (4) to (22) each containing 20% by mass of platinum.
manufacture example 4
[0226] Operation 4-1. Modulation of composite particles
[0227] The powder (1a) obtained in the process of operation 1-1 of Production Example 1 was prepared again.
[0228] The powder (1a) was put into a tubular furnace, and the furnace was heated at a heating rate of 10°C / min under a mixed gas atmosphere of hydrogen and nitrogen containing 4% by volume of hydrogen, which was introduced without passing through a bubbler containing distilled water. The internal temperature was raised to 900°C, and heat treatment was performed at 900°C for 1 hour. The heat-treated powder was naturally cooled to room temperature, pulverized in isopropanol with a planetary ball mill, filtered and dried to obtain powder (4b).
[0229] Operation 4-2. Hydrogen peroxide treatment
[0230] Except having changed the powder (1b) into 1.6g of powder (4b), it carried out similarly to operation 1-2 of manufacture example 1, and obtained the powder (henceforth "catalyst (4)").
[0231] Operation 4...
PUM
| Property | Measurement | Unit |
|---|---|---|
| specific surface area | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com