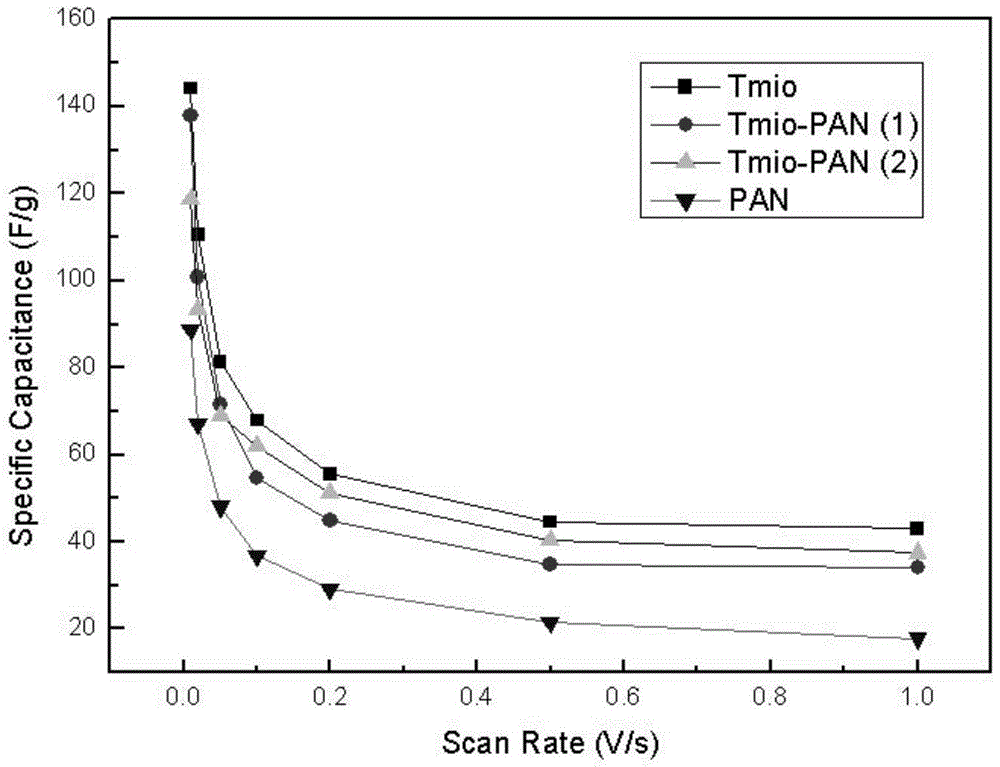
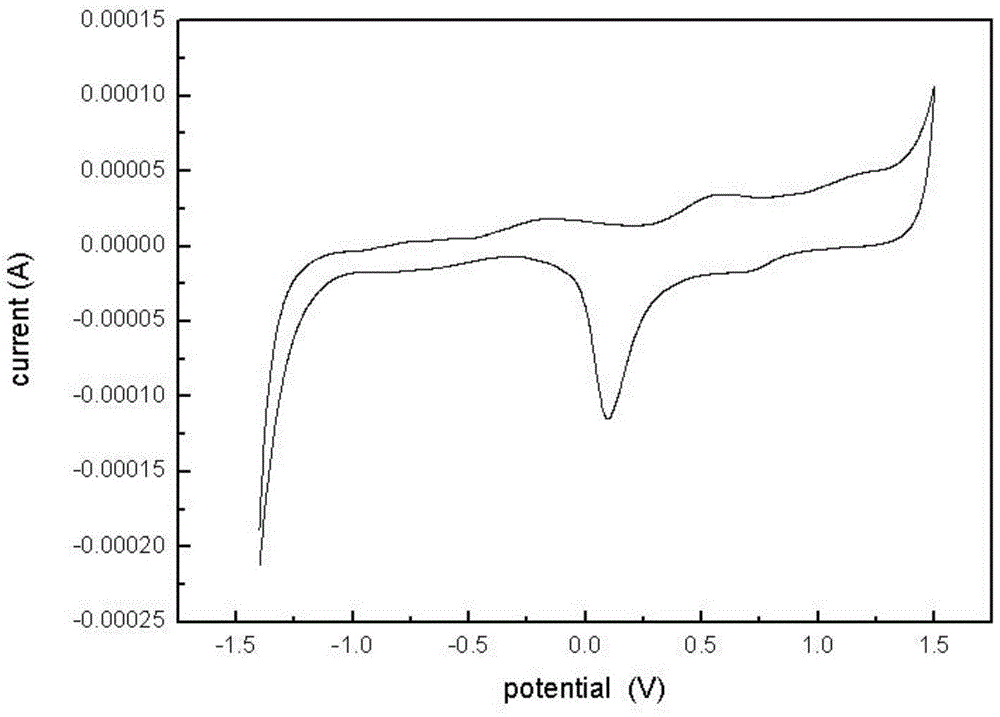
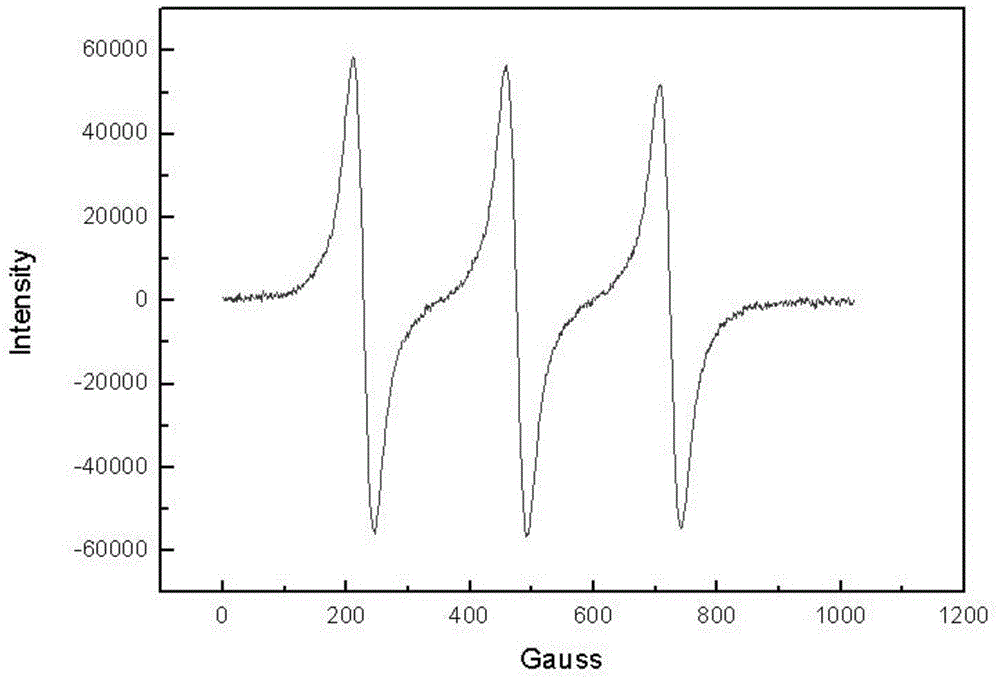
Polyaniline modified by isoazaindene nitrogen oxide free radical and its synthesis method and application
An indene nitrogen oxide and free radical technology, applied in the fields of materials and chemistry, can solve the problems of difficult energy density and cycle stability, poor solubility and processing performance, and low charge-discharge specific capacity, and achieve good dissolution and processing performance, The effect of fast electrode response, high power density
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0041] Poly(2-isozaindene nitroxide radical aminoaniline)
[0042] Stir the three-neck flask containing 20mL of water and 20mL of ethanol at 0°C, add 2 drops of concentrated hydrochloric acid, add freshly distilled aniline (4.5mL, 50mmol) under mechanical stirring, and then add the aqueous solution of ammonium persulfate drop by drop (12.18g, 53.2mmol, 60mL of water), the addition was completed after 1h, the reaction was continued for 24h, suction filtered, and vacuum dried at 40°C for 12h to obtain the polyaniline product.
[0043] Add 0.1085g of aniline to a 30mL aqueous solution of sodium hydroxide (0.2g, 5mmol), stir mechanically for 1h to remove doping, filter with suction, add the filter cake directly to 40mL of N-methylpyrrolidone, dissolve Add NaOH (0.6902g, 17.25mmol) and add p-toluenesulfonyl chloride ethanol solution (0.2579g, 30mL ethanol) drop by drop under stirring, continue the reaction for 24h, filter with suction, and dry under vacuum at 40°C to obtain toluene...
Embodiment 2
[0048] (2-Isoazaindene nitric oxide radical aminoaniline)-aniline copolymer
[0049] Add aniline (9.3g, 0.1mol) and o-nitroaniline (13.8g, 0.1mol) into a mixture of 20mL of concentrated sulfuric acid (0.4mol / L) and ethanol, dissolve, add ammonium persulfate (2.28 g, 0.1mol) of sulfuric acid solution was added to the reaction liquid to react for 2h, reacted at room temperature for 2g, filtered with suction, washed with water and ethanol several times, soaked the solid in hydrazine hydrate, filtered with suction, washed with water and ethanol several times, and dried in vacuum Obtain the copolymer product of aniline and o-nitroaniline. 0.15 g of the copolymer was added to 20 mL of hydrochloric acid, tin (1 g, 8 mmol) was added to the reaction solution, reacted in an ice bath for 12 h, filtered, added saturated sodium bicarbonate to adjust the pH to 8, removed water under reduced pressure, and dried in vacuo to obtain aniline and o- An aminoaniline copolymer product.
[0050] A...
Embodiment 3
[0054] Poly(2-isozaindene nitroxide radical vinylaniline)
[0055] Stir the three-neck flask containing 10ml of water and 10mL of ethanol at 0°C, add 1 drop of concentrated hydrochloric acid, add freshly distilled o-methylaniline (2.65mL, 25mmol) under mechanical stirring, and then add persulfuric acid drop by drop Aqueous solution of ammonium (6.10g, 26.6mmol, 30mL of water) was added after 1h, the reaction was continued for 24h, suction filtered, and vacuum dried at 40°C for 12h to obtain o-methyl polyaniline product.
[0056] O-methyl polyaniline (10.7 g, 0.1 mol) was dissolved in carbon tetrachloride (100 mL), and NBS (20 g, 0.11 mol) and catalyst BPO (0.4 g) were added to the reaction flask. Dry under nitrogen protection, reflux for 2 hours, cool to room temperature, filter, wash the filtrate three times with water, dry over anhydrous magnesium sulfate, filter, and remove the solvent in vacuo to obtain the brominated o-methylene polyaniline product.
[0057] Brominated o...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com