Preparation of n-pyridylperylenetetracarboximide and its photocatalytic hydrogen production performance
A technology of catalytic decomposition, phenyl, applied in physical/chemical process catalysts, organic compound/hydride/coordination complex catalysts, hydrogen, etc., can solve problems, limit insect pest control drug selectivity, serious resistance and other problems
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
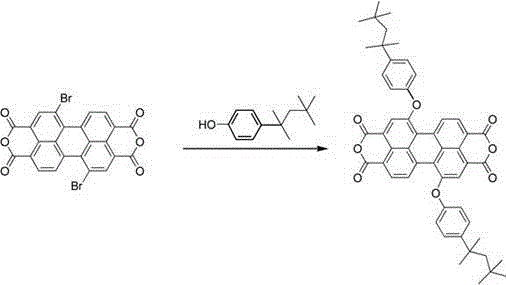
[0020] Synthesis of 1, 7-bis(4-tert-octylphenoxy)-3,4,9,10-perylene tetraanhydride
[0021]
[0022] Add 150 mL of DMF to a 250 mL three-necked flask, then add 4.01 g of 1,7-dibromoperylene anhydride, 6.01 g of octylphenol, and 4.75 g of CsCO 3 , under the protection of argon, the reaction was heated to reflux. TCL traced the disappearance of the reaction raw materials, and the reaction was completed after 5 hours, and the heating was stopped. Cool to room temperature, add 80 mL of glacial acetic acid, and continue stirring at room temperature for 1 hour. The resulting solution was frozen to precipitate a solid, suction filtered and washed several times with cold methanol, and the resulting solid was dried;
[0023] (2) Synthesis of POPPDA
[0024]
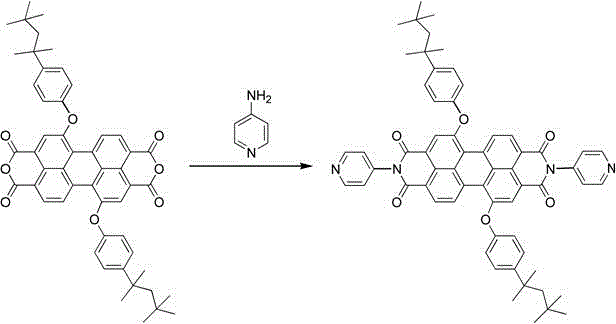
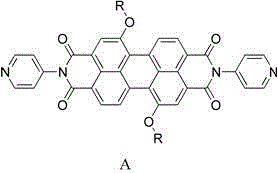
[0025] Weigh 0.48g of the above-mentioned dried solid and place it in a 100mL three-neck flask, add 20mL of dried quinoline, then add 0.19g of 4-aminopyridine, 0.07g of zinc acetate, pass in argon, and heat to reflux for ...
Embodiment 2
[0029] Diacetyl oxime cobalt complex [Co(DH)(DH 2 )Cl 2 ]Synthesis:
[0030] Add 25 g (0.105 mol) cobalt chloride hydrate, 750 mL acetone and 24.5 g (0.211 mol) dimethylglyoxime into a 250 mL three-neck flask, stir the mixture for 10 min, and filter to remove insoluble matter. The resulting mother liquor was allowed to stand overnight, and green crystals were precipitated, filtered, washed with acetone, and dried to obtain 28.5 g (76%) of the product.
Embodiment 3
[0032] Pyridyl dimethylglyoxime cobalt complex [(py)Co(DH)(DH 2 )Cl] synthesis:
[0033] The dimethylglyoxime cobalt complex (3.3 g, 0.009 mol) synthesized in Example 2 was suspended in 85 mL of chloroform solvent, and pyridine (1.8 g) was added to the solution and shaken. After reacting for 5 minutes, 30 mL of water was added, vigorously stirred for 2 hours, and the water layer was removed. The chloroform layer was washed 3 times with water, most of the chloroform was spin-dried, and 95% ethanol solution was added to precipitate a solid, which was recrystallized using dichloromethane ethanol solvent to obtain brown crystals with a yield of 60%. Anal. Calcd for C 13 ClCoH 19 N 5 o 4 : C, 38.9; H, 4.7; Co, 14.6, Found: C, 38.8; H, 5.0; Co, 14.7.
PUM
 Login to view more
Login to view more Abstract
Description
Claims
Application Information
 Login to view more
Login to view more - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap