Modified polysaccharide with fluorescent property as well as preparation method and application thereof
A polysaccharide and modification technology, applied in the field of polysaccharide-based fluorescent materials and their preparation, can solve the problems of poor fluorescence stability, change of material fluorescence properties, and difficulty in uniform dispersion of fluorescent molecules, and achieve easy processing and molding, high fluorescence intensity, and elimination of fluorescence. The effect of the aggregation quenching effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
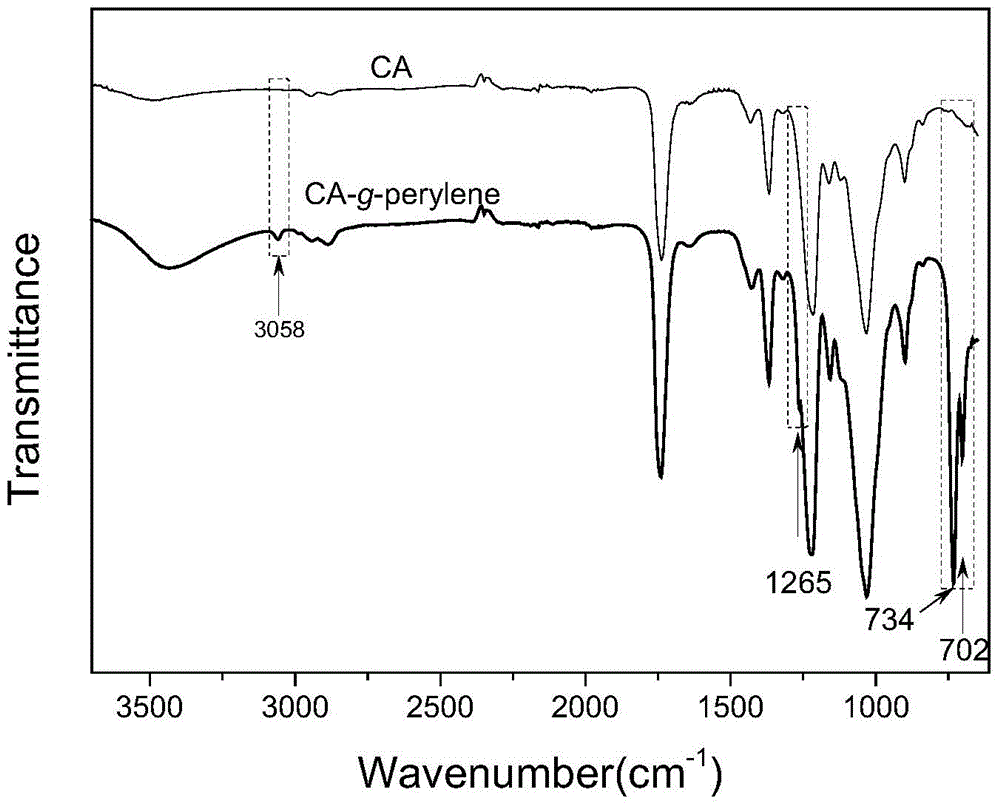
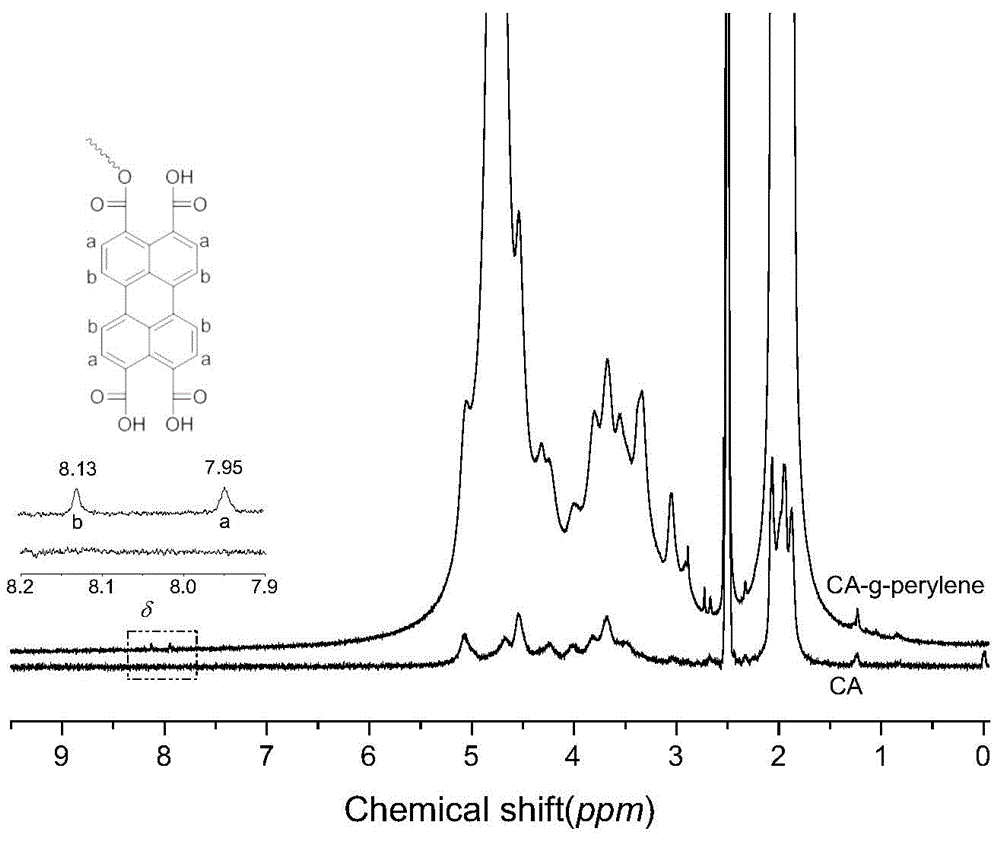
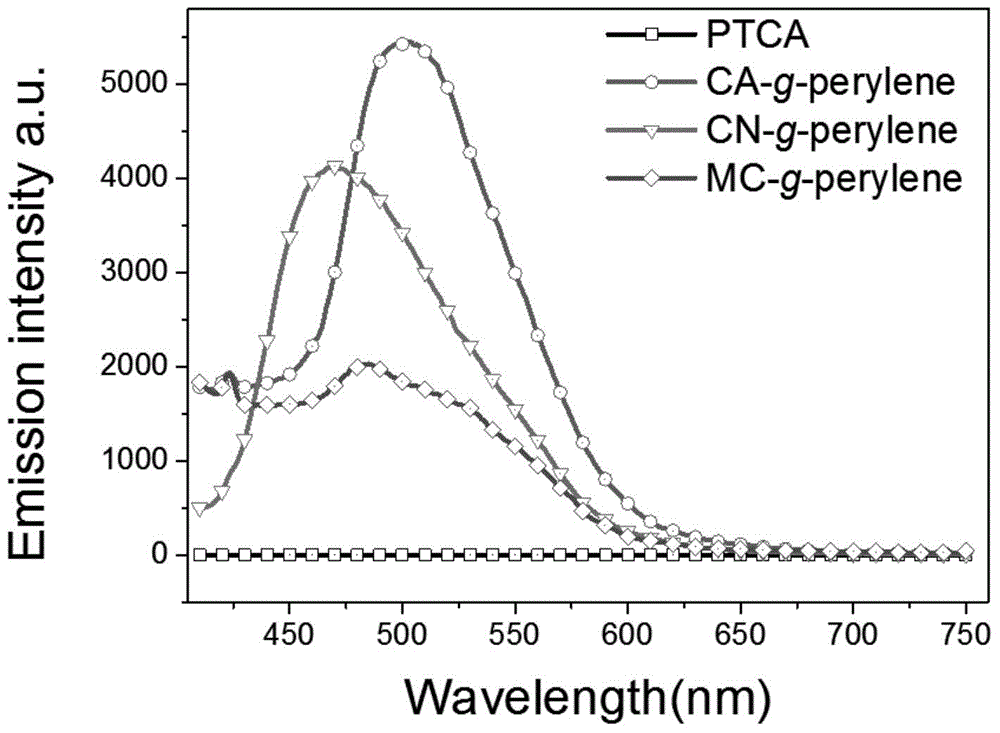
[0057] 0.47g of cellulose acetate (CA), 0.004g of perylenetetracarboxylic acid (PTCA), 0.006g of DMAP, and 0.25g of DCC were dissolved in 250mL of DMF solution, and refluxed for 1 hour in an oil bath at 150°C. After the reaction was finished, after most of the DMF was removed by rotary evaporation, the concentrated reaction solution was added to methanol, and after precipitation, washing and drying, the perylene-containing cellulose acetate derivative (referred to as CA-g-perylene ).
Embodiment 2
[0059] 0.46g of ethylcellulose (MC), 0.05g of perylenetetracarboxylic acid, 0.006g of DMAP, and 0.25g of DCC were dissolved in 250mL of DMF solution, and refluxed for 1 hour in an oil bath at 150°C. After the reaction is completed, after most of the DMF is removed by rotary evaporation, the concentrated reaction solution is added to about 100mL of 0.001mol / L sodium hydroxide solution, and the perylene ethyl cellulose with a degree of substitution of 0.032 can be obtained after precipitation, washing and drying. Ester derivatives (referred to as MC-g-perylene).
Embodiment 3
[0061] 0.53g of nitrocellulose (CN), 0.05g of perylenetetracarboxylic acid, 0.006g of DMAP, and 0.25g of DCC were dissolved in 250mL of DMF solution, and refluxed for 0.8h in an oil bath at 150°C. After the reaction was completed, after most of the DMF was removed by rotary evaporation, the concentrated reaction solution was added to a mixed solution of about 150mL sodium hydroxide (0.001mol / L) and methanol (v / v, 4:1), and precipitated, washed, After drying, a perylene-containing nitrocellulose ester derivative (referred to as CN-g-perylene) with a degree of substitution of 0.012 can be obtained.
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com