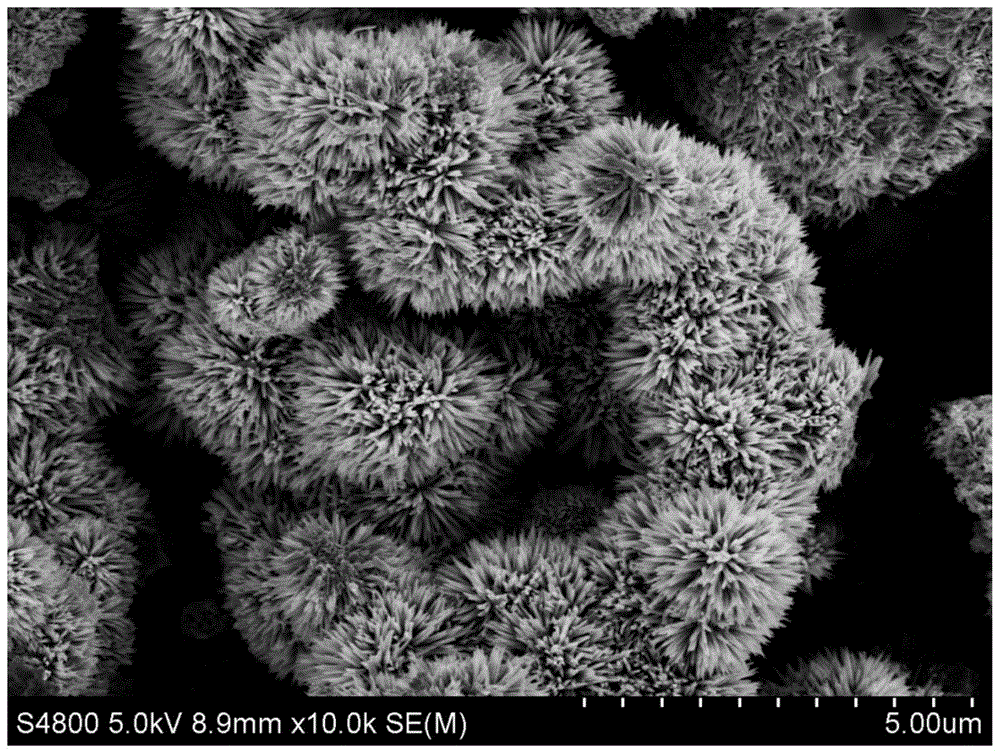
Catalyst for CO selective hydrogenation and its preparation and application
A catalyst and selective technology, applied in chemical instruments and methods, preparation of hydroxyl compounds, preparation of organic compounds, etc., can solve the problems of less research on nanomaterials, and achieve good dispersion, easy recycling, uniform shape and size Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0042] Weigh 0.75g cobalt acetate (Co(Ac) 2 ·4H 2 O) was dissolved in 70 mL of 1,2-propanediol to form solution a.
[0043] Weigh 20 mg of palladium nitrate (Pd(NO 3 ) 2 ·H 2 O) was dissolved in 20 ml of 1,2-propanediol to form solution b.
[0044] Take 0.5ml of solution b and add it to solution a, stir and dissolve at room temperature to obtain a uniform solution A.
[0045] Then 1.05 g of stearic acid was weighed, added to solution A, stirred at 40° C. for 30 min, and mixed evenly to obtain solution B.
[0046] The obtained solution B was added to a 100 ml polytetrafluoroethylene hydrothermal kettle, and hydrothermally reacted for 5 hours under airtight conditions at a temperature of 190°C. Then, turn off the heating, take out after the reaction temperature is cooled to room temperature, and it can be observed that the upper layer is a golden yellow colloidal solution, and there is a certain amount of black solid product on the inner wall of the reactor, scrape out the...
Embodiment 2
[0050] Weigh 0.75g cobalt acetate (Co(Ac) 2 ·4H 2 O) was dissolved in 70 mL of propylene glycol to form solution a.
[0051] Weigh 200 mg of palladium nitrate (Pd(NO 3 ) 2 ·H 2 O) was dissolved in 20 ml of propylene glycol to form solution b.
[0052] Take 0.5 ml of solution b and add it to solution a, and stir and dissolve to obtain a uniform solution A at normal temperature.
[0053] Then 1.05 g of stearic acid was weighed, added to solution A, stirred at 40° C. for 30 min, and mixed evenly to obtain solution B.
[0054] The obtained solution B was added to a 100 ml polytetrafluoroethylene hydrothermal kettle, and hydrothermally reacted for 5 hours under airtight conditions at a temperature of 190°C. Then, turn off the heating, take out after the reaction temperature is cooled to room temperature, and it can be observed that the upper layer is a golden yellow colloidal solution, and there is a certain amount of black solid product on the inner wall of the reactor, scra...
Embodiment 3
[0057] Weigh 0.75g cobalt acetate (Co(Ac) 2 ·4H 2 O) was dissolved in 70 mL of propylene glycol to form solution a.
[0058] Weigh 200mg copper acetate (Cu(Ac) 2 ·H 2 O) was dissolved in 20 ml of propylene glycol to form solution b.
[0059] Take 2.5ml of solution b and add it to solution a, under normal temperature, stir and dissolve to obtain a uniform solution A.
[0060] Then 1.05 g of stearic acid and 1.05 g of hexadecylamine were weighed, added to solution A, stirred at 40° C. for 30 min, and mixed evenly to obtain solution B.
[0061] The obtained solution B was added to a 100 ml polytetrafluoroethylene hydrothermal kettle, and hydrothermally reacted for 5 hours under airtight conditions at a temperature of 190°C. Then, turn off the heating, take out after the reaction temperature is cooled to room temperature, and it can be observed that the upper layer is a golden yellow colloidal solution, and there is a certain amount of black solid product on the inner wall of...
PUM
| Property | Measurement | Unit |
|---|---|---|
| length | aaaaa | aaaaa |
| diameter | aaaaa | aaaaa |
| diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com