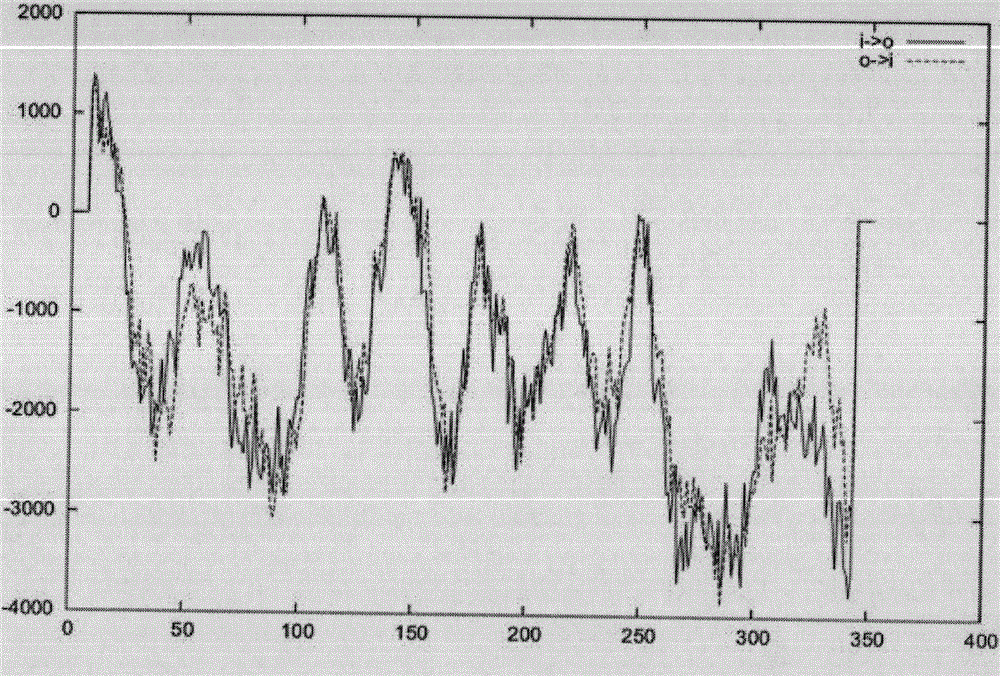
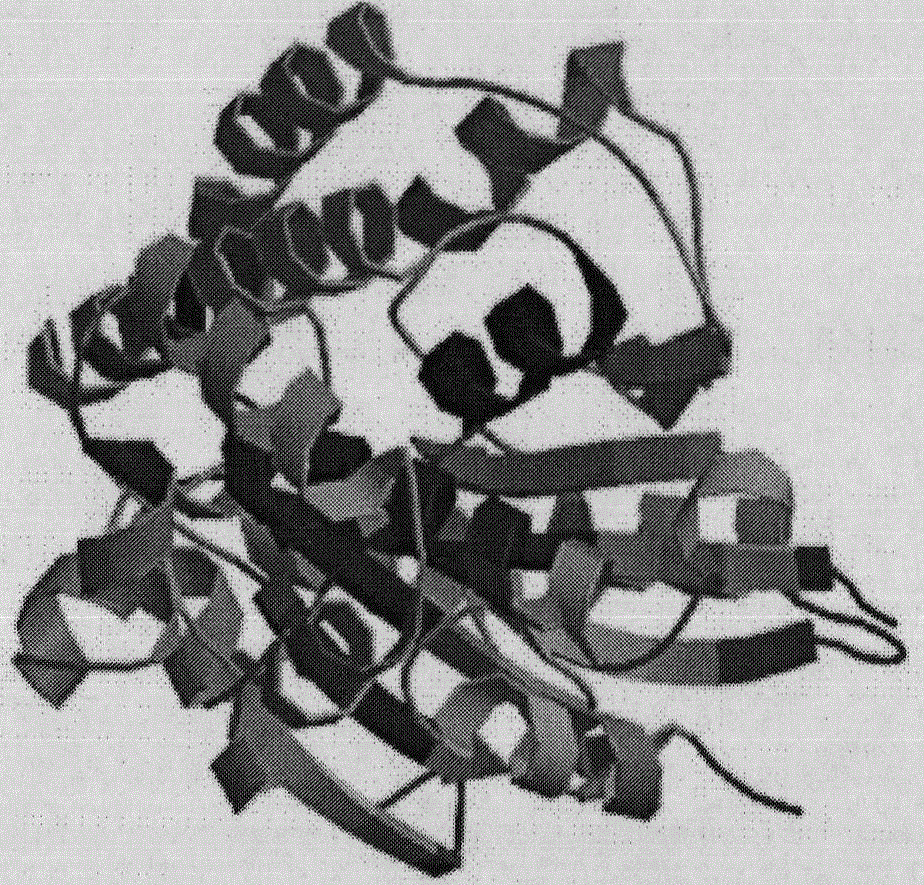Key enzyme MCAT in synthesis path of Nannochloropsis gaditana fatty acid FAS II
A technology for fatty acid synthesis and Nannochloropsis, which is applied in the field of enzyme proteins, can solve the problems of blocked protein synthesis, lack of related enzymes, and reduced overall cell activity, and achieves the effect of improving the efficiency of oil accumulation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0021]The experiment selected Nannochloropsis gaditana HH-1 from the algal species library of the First Institute of Oceanography, State Oceanic Administration. Competent cells (E.coli) were purchased from Quanshijin Company, Escherichia coli strain DH5α was preserved in our laboratory, Saccharomyces cerevisiae strain and pYES2 expression plasmid were purchased from Invitroen Company; primer synthesis and sequencing were carried out in Shanghai Sangong. For the experimental methods that do not indicate specific conditions in the following examples, conventional conditions can usually be used, such as the conditions described in the "Molecular Cloning Experiment Guide" written by J. Sambrook (Sambrook), or according to the manufacturer's suggestion conditional run. Embodiment 1: Acquisition of full-length cDNA of the key enzyme MCAT encoding protein in the FAS type II fatty acid synthesis pathway of the present invention
[0022] In the present invention, firstly, the whole ge...
Embodiment 2
[0031] Example 2: Bioinformatics analysis of the key enzyme MCAT in the FAS type II fatty acid synthesis pathway
[0032] The open reading frame analysis of Ng-MCAT gene was carried out by NCBI; the theoretical molecular weight and isoelectric point of the protein were predicted by ProtParam software; the TmPred algorithm software was used to analyze and predict the transmembrane region of the protein; the third level of Ng-MCAT protein was predicted by SWIMM-MODEL Structure; finally, compare the determined Ng-MCAT sequence with the MCAT genes of other species known in GenBank with BLAST software, select sequences with higher similarity and use Bioedit software for alignment sorting, and construct a phylogenetic tree; use Bioedit software Amino acid sequence homology analysis was carried out between Ng-MCAT and MCAT of several algae with close evolutionary relationship.
[0033] Using GeneDoc software, the Ng-MCAT amino acid sequence was homologously compared with the MCAT ami...
Embodiment 3
[0034] Embodiment 3: MCAT Saccharomyces cerevisiae expression vector construction
[0035] Construction and main structure of pYES2-MCAT recombinant plasmid Figure 4 shown
[0036] Digest Ng-MCAT and shuttle plasmid pYES2 with BamH I and XbaI, connect with T4 DNA ligase to obtain plasmid pYES2, transform Escherichia coli DH5α, extract a small amount of plasmid DNA and digest it with TBamH I and XbaI, and detect the digested products by electrophoresis , excised a fragment of about 1059bp, indicating that Ng-MCAT was connected to the expression plasmid pYES2. The Ng-MCAT gene was amplified by PCR with the designed primers, and the amplification result was detected by electrophoresis. The amplified fragment of Ng-MCAT was about 1059 bp, which was in line with the expected size.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to view more
Login to view more - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap