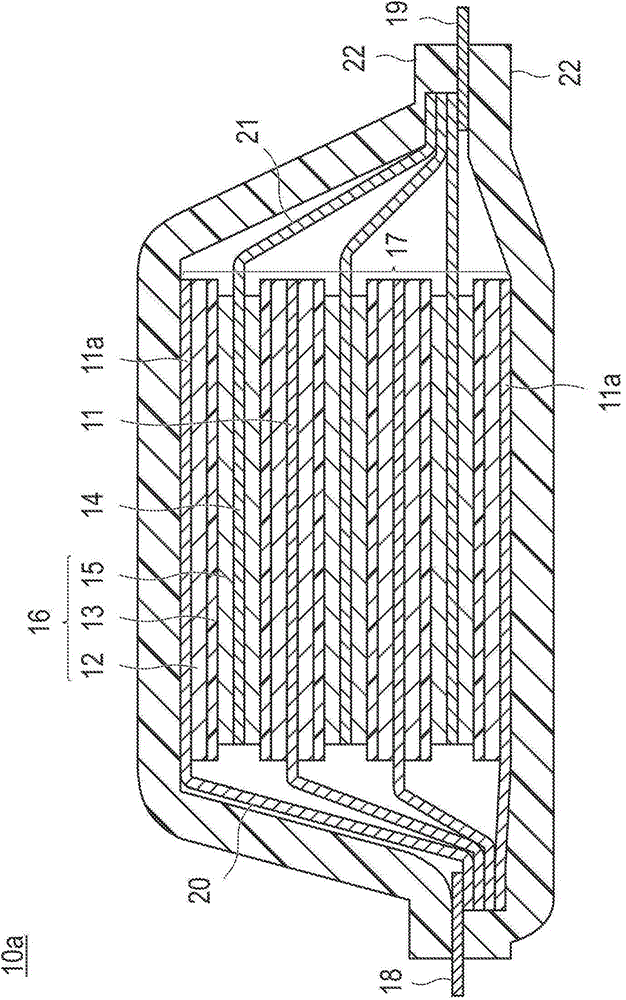
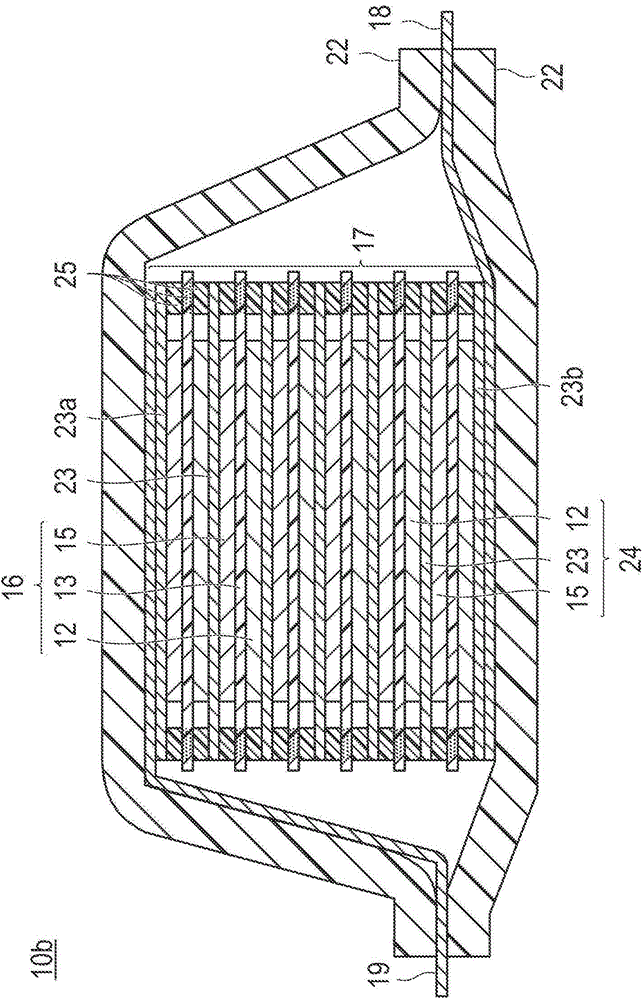
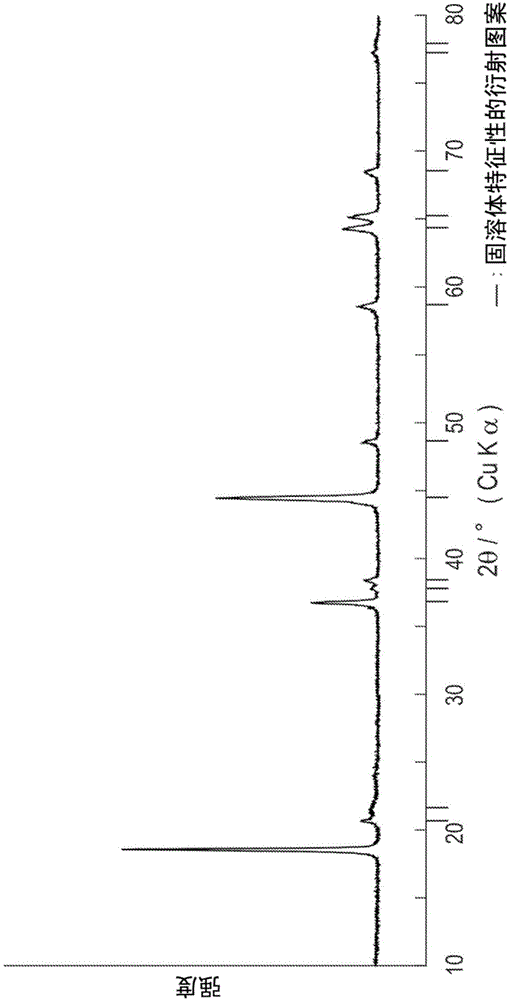
Positive electrode active substance for nonaqueous electrolyte secondary cell, method for producing positive electrode active substance for nonaqueous electrolyte secondary cell, and nonaqueous electrolyte secondary cell
A positive active material, non-aqueous electrolyte technology, applied in non-aqueous electrolyte storage batteries, active material electrodes, secondary batteries and other directions, can solve the problem of non-aqueous electrolyte secondary battery capacity reduction and other issues
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0146] (Preparation of titanium-citric acid complex aqueous solution)
[0147] Add 60g (0.3mol) of citric anhydride (molecular weight: 192.12g / mol) into 400ml of acetone, and heat to 60°C for dissolution. Then, 56 g (0.2 mol) of titanium tetraisopropoxide (molecular weight 284.22 g / mol) was added to form a precipitate. The liquid was filtered with suction to obtain a precipitate (pale yellow).
[0148] Add H to the precipitate 2 O (200ml), heated to 50-60°C to dissolve. The solution was left to stand for more than one day to settle the insoluble matter, and then filtered to remove the insoluble matter to obtain a titanium-citric acid complex aqueous solution. Ti concentration as TiO 2 (Molecular weight: 79.87 g / mol) was calculated as 5.0 weight%.
[0149] (Preparation of positive electrode active material C1) Li 1.5 [Ni 0.375 mn 0.775 [Li] 0.25 Ti 0.10 ]O z
[0150] To 15.97g titanium-citric acid complex aqueous solution (with TiO 2 Add 15.93g manganese acetate te...
Embodiment 2
[0202] (Preparation of positive electrode active material C2) Li 1.5 [Ni 0.375 mn 0.775 [Li] 0.25 Ti 0.10 ]O z
[0203] To 15.97g titanium-citric acid complex aqueous solution (using TiO 2 Add 15.93g manganese acetate tetrahydrate (molecular weight 245.09g / mol), 6.22g nickel acetate tetrahydrate (molecular weight 248.84g / mol), 1.01 times the amount of 15.31g (15.46g) Lithium acetate dihydrate (molecular weight: 102.02 g / mol). The obtained mixture is heated to 200°C to 300°C to melt and dissolve. Then, using a spray drying apparatus, the obtained molten solution (slurry) is heated and sprayed at 200°C to 400°C, and dried. The obtained dry powder was calcined at 900° C. for 12 hours after pre-calcining at 600° C. for 4 hours. Add 10g of calcined powder into 100ml of pure water, stir and mix for two minutes, then quickly filter with suction. After drying the filtrate at 200°C, it was baked at 400°C for 1 hour.
[0204] The composition of the positive electrode active m...
Embodiment 3
[0209] The positive electrode active material C3 was prepared according to Example 2. In addition, an evaluation battery cell was manufactured according to Example 1, and after the initial charge treatment and activation treatment, performance evaluation and lifetime evaluation were performed. The evaluation results are shown in Table 1 and Table 3 below.
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
| specific surface area | aaaaa | aaaaa |
| density | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com