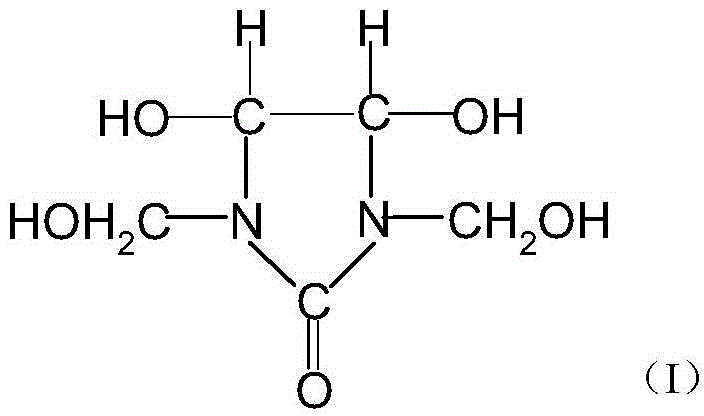
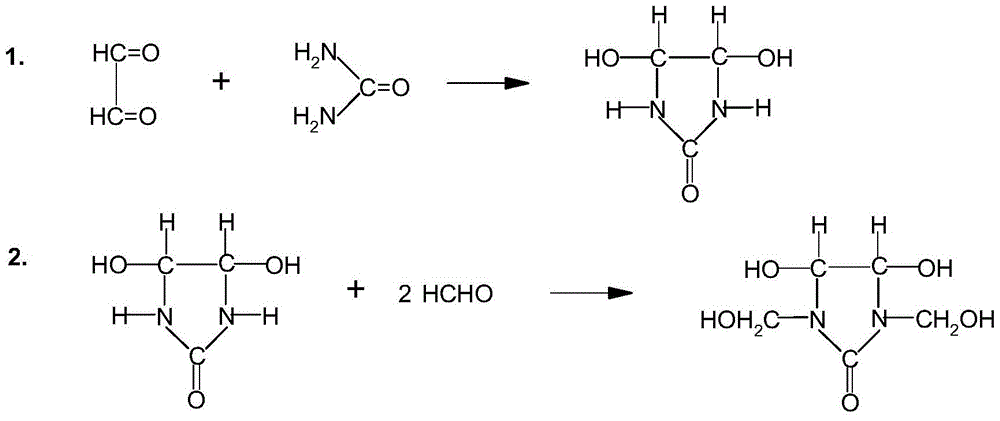
Improved preparation method of dimethylol dihydroxyethylene urea
A technology of dimethylol dihydroxyethylene urea and dihydroxyethylene urea, which is applied in the field of textile functional finishing, can solve the problems affecting the etherification modification reaction of products, the conversion rate of glyoxal, the stability of high-concentration products and the reactivity, etc. Rarely involve problems such as poor storage stability, etc., to achieve the effects of high conversion rate, good stability and stable reaction
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
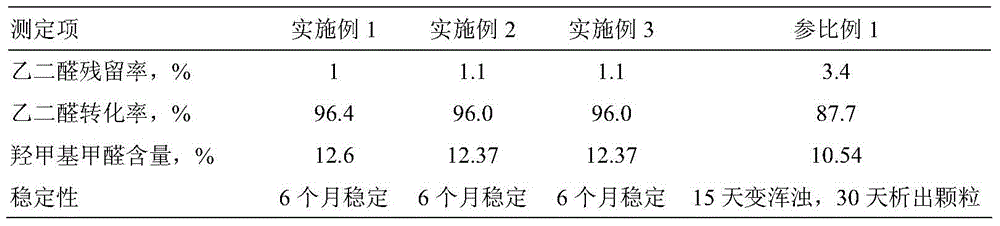
[0053] In a 1-liter four-necked round-bottomed flask equipped with a stirrer, a thermometer and a condenser tube, add 290 grams of glyoxal (40% content) and then 126 grams of urea (99% content), stirring and dissolving at 35°C. Then add 0.12g of methanesulfonic acid (content 70%), 0.60g of sodium methanesulfonate (content 98%), and react at 40°C for 2 hours; then add 0.20g of sodium methanesulfonate, heat up to 55°C, The reaction was continued for 2 hours. Determination of residual glyoxal (results in Table 1).
[0054] Then the reactant was cooled to 50°C, 2.6 g of disodium hydrogen phosphate and 0.16 g of sodium dihydrogen phosphate were added, and a mixture of 300 g of formaldehyde solution (37%) and 2.5 g of dimethylaminoethanol (dimethylaminoethanol) was added dropwise within 1-2 hours Add aminoethanol to the formaldehyde solution in advance), and continue the reaction at 50°C for 4 hours to obtain a light yellow transparent liquid. Determination of active group methylo...
Embodiment 2
[0057] In a 1L four-necked round-bottomed flask equipped with a stirrer, a thermometer and a condenser tube, add 290 grams of glyoxal (content 40%), then add 130 grams of urea (content 99%), stir and dissolve at 35 °C. Then add 0.21g of ethylsulfonic acid (content 70%), sodium ethylsulfonate 0.82g (content 98%), and react at 40°C for 2 hours; then add 0.50g of sodium ethylsulfonate, heat up to 50°C, The reaction was continued for 2 hours. Determination of residual glyoxal (results in Table 1).
[0058] Then add 2.8 g of disodium hydrogen phosphate, 0.20 g of sodium dihydrogen phosphate, and dropwise add 300 g of formaldehyde solution (37%), 2.5 g of dimethylaminoethanol, and 1.5 g of diethylaminopropanol (dimethylaminopropanol) for 1-2 hours. Add aminoethanol and diethylaminopropanol to the formaldehyde solution in advance), and continue the reaction at 55°C for 3 hours to obtain a light yellow transparent liquid. Determination of active group methylol formaldehyde content (...
Embodiment 3
[0061] In a four-necked round-bottomed flask equipped with a stirrer, a thermometer and a condenser tube, add 290 grams of glyoxal (content 40%), then add 130 grams of urea (content 99%), stir and dissolve at 35°C. Then add 0.30g of benzenesulfonic acid (content 80%), 0.80g of sodium benzenesulfonate (content 98%), and react for 2 hours at 40°C; then add 0.45g of sodium benzenesulfonate, heat up to 50°C, and continue the reaction for 2 hours Hour. Determination of residual glyoxal (results in Table 1).
[0062] Then add 2.8g of dipotassium hydrogen phosphate, 0.20g of potassium dihydrogen phosphate, and dropwise add 300g of formaldehyde solution (37%), 2.5g of dimethylaminoethanol, and 1.5g of diethylaminopropanol (dimethylaminopropanol) for 1-2 hours. Add aminoethanol and diethylaminopropanol to the formaldehyde solution in advance), and continue the reaction at 55°C for 3 hours to obtain a light yellow transparent liquid. Determination of active group methylol formaldehyde...
PUM
| Property | Measurement | Unit |
|---|---|---|
| refractive index | aaaaa | aaaaa |
| refractive index | aaaaa | aaaaa |
| refractive index | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com