A kind of preparation method of azoxystrobin intermediate
An intermediate, azoxystrobin technology, applied in the field of preparation of pesticide intermediates, can solve the problems of long reaction time, high production cost, and difficult purification of impurities, and achieve the effects of easy purification, improved purity, and reduced production cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
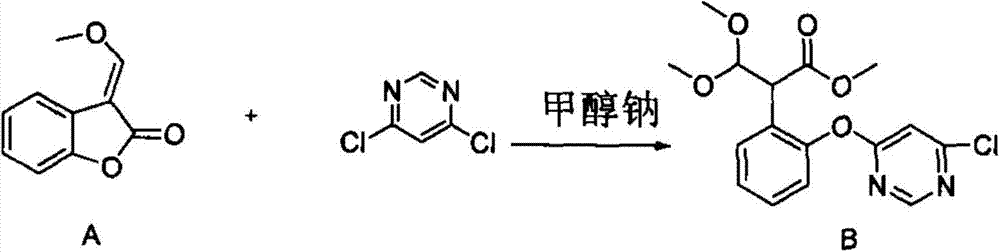
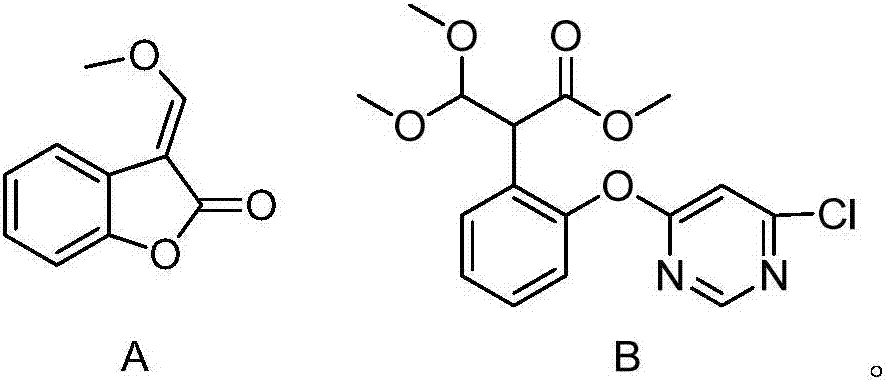
[0019] 46.6 grams of compound A (98%, 0.26mol), 46.6 grams of dichloropyrimidine (98.5%, 0.308mol) and 130 milliliters of tetrahydrofuran were successively dropped into a 500 milliliter three-necked flask, stirred evenly at room temperature, and then dropped into 2-methyldivinylpiperene 0.4 g of oxazine, stirred and cooled to 15° C., began to dropwise add 53.8 grams of methanol solution of sodium methoxide (28.87%, 0.287 mol), the dropwise addition time was controlled for 5 hours, and the dropwise addition was completed and kept at 20° C. for 1 hour. Add 36% hydrochloric acid to acidify to PH = 4, add water, stir and wash twice, and desolventize to obtain the crude compound B with a yield of 80%.
Embodiment 2
[0021] 46.6 grams of compound A (98%, 0.26mol) and 130 milliliters of acetonitrile were successively dropped into a 500 milliliter three-necked flask, stirred evenly at room temperature, and then 0.4 grams of 2-methyldivinylpiperazine was dropped into, stirred and cooled to 15° C., and 53.8 Methanol solution of gram sodium methylate (28.87%, 0.287mol), dropwise time control half an hour, 20 ℃ of insulations 1 hour after dropwise finishing, add 46.6 gram dichloropyrimidines (98.5%, 0.308mol) then. After heat preservation for 1 hour, 36% hydrochloric acid was added to acidify to PH=4, and water was added to stir and wash twice, and the compound B was precipitated to obtain crude compound B with a yield of 85%.
Embodiment 3
[0023] 46.6 grams of compound A (98%, 0.26mol), 46.6 grams of dichloropyrimidine (98.5%, 0.308mol) and 130 milliliters of toluene were successively dropped into a 500 milliliter three-necked flask, stirred evenly at room temperature, and then dropped into 2-methyl divinylpiperidine 0.4 g of oxazine, stirred and cooled to 15° C., began to dropwise add 53.8 grams of methanol solution of sodium methoxide (28.87%, 0.287 mol), the dropwise addition time was controlled for 5 hours, and the dropwise addition was completed and kept at 20° C. for 1 hour. Add 36% hydrochloric acid to acidify to PH = 4, add water, stir and wash twice, and desolventize to obtain the crude compound B with a yield of 90%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com