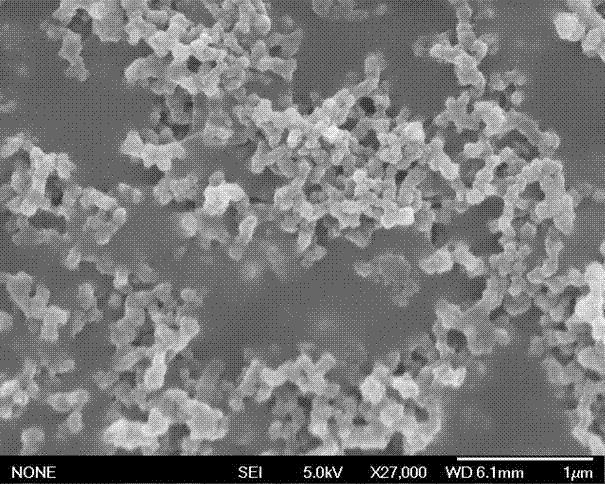
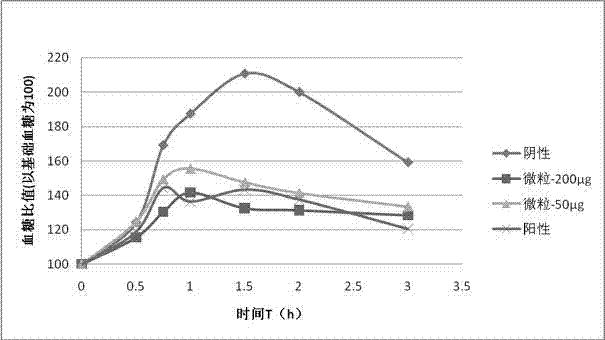
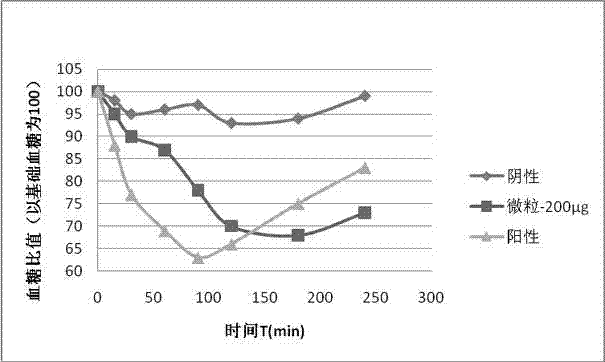
Exenatide oral preparation and preparation method thereof
A technology of exenatide and oral preparations, which is applied in the field of exenatide oral preparations and its preparation, can solve the problems of patients' physical, psychological and economic burdens, poor patient compliance, and low bioavailability, so as to improve bioavailability , lowering blood sugar, lowering the effect of fasting blood sugar
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0041] Example 1 Preparation of exenatide-charged excipient particles
[0042] S1. Take a certain amount of charged auxiliary chitosan (molecular weight: 20,000-100,000) and place it in 5% (v / v) glacial acetic acid aqueous solution to fully dissolve it so that the concentration of chitosan is 4 mg / ml ( m / v); configure exenatide aqueous solution so that the concentration is 8 mg / ml;,
[0043] S2. measure the chitosan solution of 10ml in the vial, add 2ml exenatide solution and stir, add cross-linking agent sodium tripolyphosphate after 3h, stir for 24h, get exenatide-chitosan microparticle solution;
[0044] S3. Stop stirring, remove the supernatant, and centrifuge at 15,000 rpm for 5 minutes; add the lyoprotectant lactose, freeze-dry, and exenatide-chitosan microparticles.
Embodiment 2
[0045] Example 2 Preparation of exenatide-charged excipient particles
[0046] S1. Take a certain amount of charged auxiliary material acrylic resin EudragitL100 and place it in a mixed solvent of methanol and dichloromethane to fully dissolve it so that the concentration of EudragitL100 is 0.5 mg / ml (m / v); prepare an aqueous solution of exenatide to make the concentration 0.5 mg / ml; The two solutions are mixed and homogeneous at 8000rpm / 1min at low temperature. The mixed solution was transferred to liquid paraffin containing Span 80, and the solvent evaporated rapidly under stirring.
[0047] S2. Wash the obtained particles with petroleum ether and ultrapure water, and filter.
[0048] S3. The microparticles were dispersed in pure water, centrifuged at 8000 rpm for 20 minutes, and the supernatant was removed; the freeze-drying protective agent mannitol was added and freeze-dried to obtain exenatide-acrylic resin microparticles.
Embodiment 3
[0049] Example 3 Preparation of exenatide-charged excipient particles
[0050] S1. Take a certain amount of charged excipient sodium alginate (molecular weight: 20,000-100,000), fully dissolve it in water, so that the concentration of sodium alginate is 2 mg / ml (m / v); configure exenatide aqueous solution, so that the concentration is 2 mg / ml;
[0051] S2. Measure 10ml of sodium alginate solution in a vial, add 0.5ml of exenatide solution and stir, add cross-linking agent glutaraldehyde after 0.5h, and stir for 12h to obtain exenatide-sodium alginate particle solution;
[0052] S3. Stop stirring, remove the supernatant, and centrifuge at 8000 rpm for 20 min; add lyoprotectant sucrose, freeze-dry, exenatide-sodium alginate microparticles.
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com