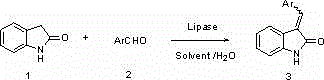Method for synthesizing 3-substituted-2-indolone compounds under catalysis of lipase
A technology of lipase and indolinone, which is applied in the field of biocatalysis, can solve the problems of condensation reactions that have not been reported in the literature, and achieve the effects of wide application range of substrates, low production costs and strong competitive advantages
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0039] Biological enzymes and other reagents involved in the present invention are purchased from the market, and wherein the reagents are not further purified; proton nuclear magnetic resonance (1HNMR) is measured with a Bruker Advance 2B 400 nuclear magnetic resonance spectrometer, and the frequency is 400MHz, and the solvent is deuterated Dimethyl sulfoxide, internal standard is tetramethylsilyl (TMS).
[0040] In all examples, lipase-catalyzed Knoevenagel condensation reaction of aromatic aldehydes and indol-2-ones, the reaction equation is:
[0041] .
example 1
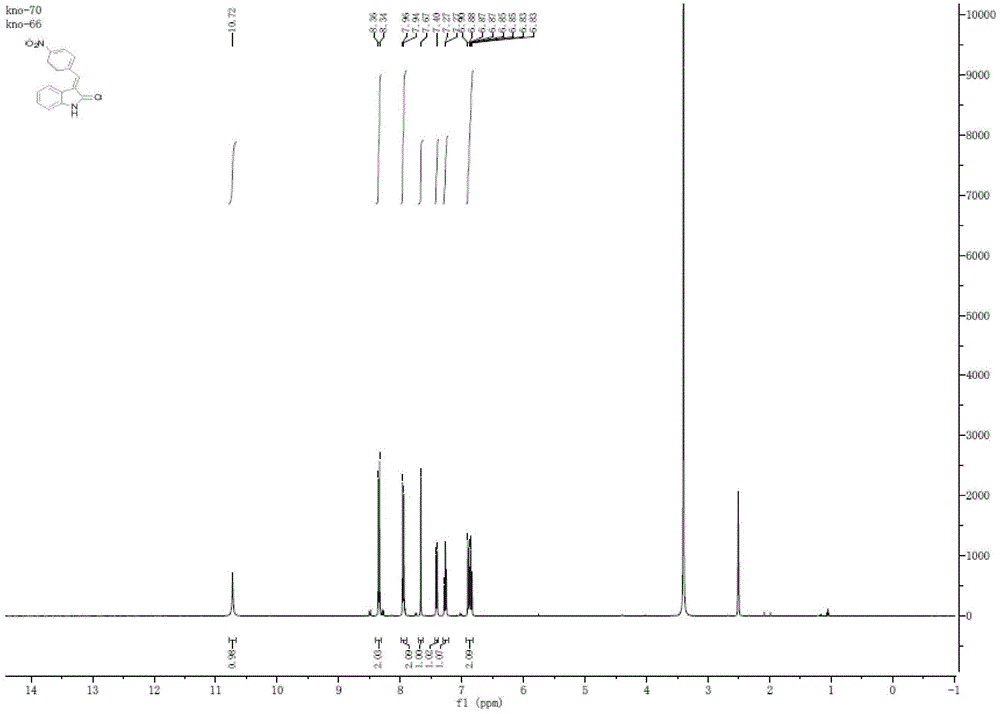
[0042] Example 1: 2 mmol p-nitrobenzaldehyde and 2 mmol indol-2-one are added in a 10 ml reaction flask, then 125 mg of porcine pancreatic lipase, 1 ml of deionized water, 4 ml of DMSO are stirred and reacted at 45° C. TLC (petroleum ether / ethyl acetate, 1 / 1, v / v) monitored the progress of the reaction. After 10 hours, filter out the enzyme, filter out the enzyme after the reaction, filter paper and filtrate with CH 2 Cl 2 Wash with water three times. The filtrate was extracted three times with 5ml of water and 10ml of ethyl acetate, the combined organic phases were washed with anhydrous MgSO 4After drying and filtering, rotary evaporation under reduced pressure, the crude product was separated by column chromatography [petroleum ether / ethyl acetate, 9 / 1-2 / 1, v / v] to obtain the purified target product with a yield of 94.2%, mp: 245–250°C. 1 H NMR (400 MHz, DMSO) δ 10.72 (s, 1H), 8.35 (d, J = 8.7 Hz, 2H), 7.95 (d, J = 8.4 Hz, 2H), 7.67 (s, 1H), 7.41 (d , J = 7.7 Hz, 1H), 7...
example 2
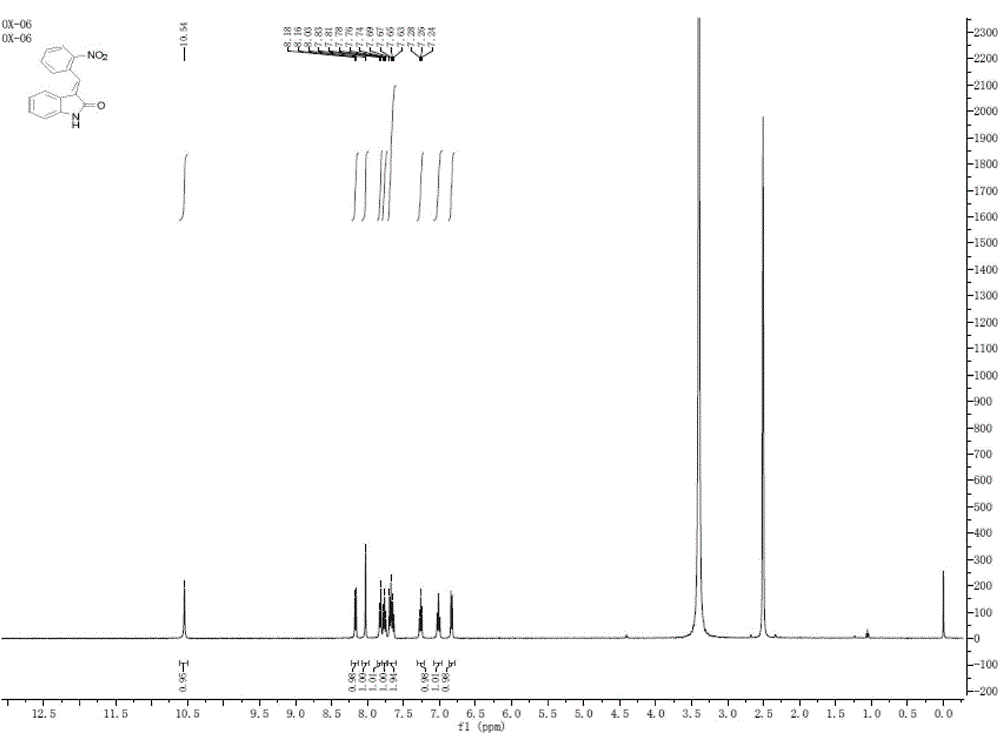
[0043] Example 2: 2 mmol o-nitrobenzaldehyde and 2 mmol indol-2-ketone are added in a 10 ml reaction flask, then 125 mg porcine pancreatic lipase, 1 ml deionized water, 4 ml DMSO are stirred at 45° C. TLC (petroleum ether / ethyl acetate, 1 / 1, v / v) monitored the progress of the reaction. After 10 hours, the enzyme was filtered out, and the enzyme was filtered out after the reaction was completed. The filter paper and the filtrate were respectively washed with CH 2 Cl 2 Wash with water three times. The filtrate was extracted three times with 5ml of water and 10ml of ethyl acetate, the combined organic phases were washed with anhydrous MgSO 4 After drying and filtering, rotary evaporation under reduced pressure, the crude product was separated by column chromatography [petroleum ether / ethyl acetate, 9 / 1-2 / 1, v / v] to obtain the purified target product with a yield of 89.2%, mp: 199-200°C. 1 H NMR (400 MHz, DMSO) δ 10.54 (s, 1H), 8.17 (d, J = 8.2 Hz, 1H), 8.03 (s, 1H), 7.86 –...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com