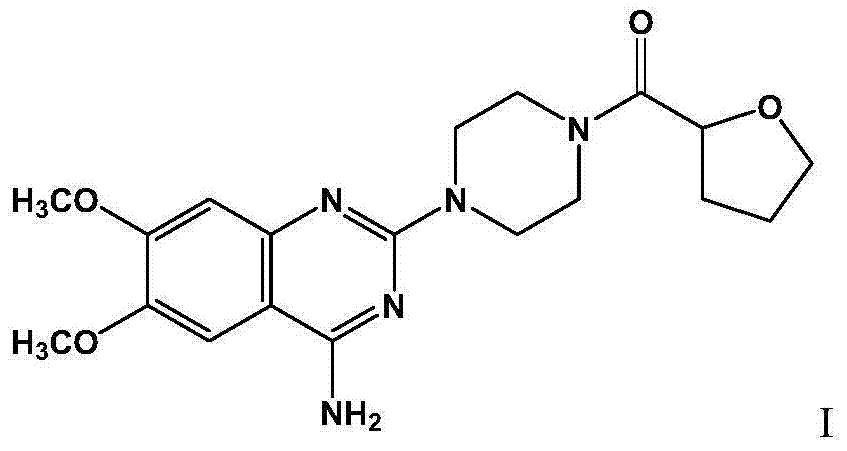
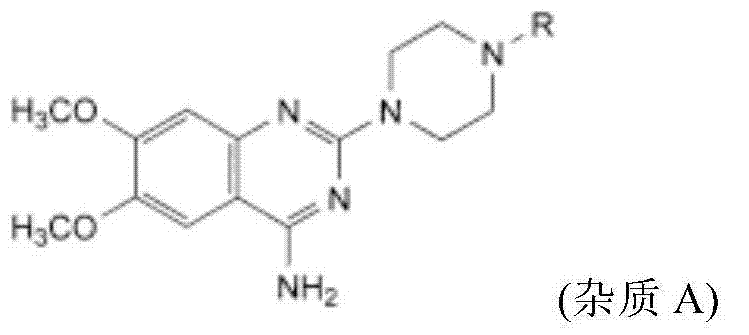
Medicine composition
A technology of composition and medicine, applied in the field of medicine
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0148] Embodiment 1: prepare terazosin freeze-dried powder injection
[0149] formula:
[0150] Terazosin: 1mg,
[0151] Mannitol: 50mg,
[0152] Acid-base regulator: use appropriate amount when necessary, and adjust the pH to the specified value in the following preparation method,
[0153] Water for injection: up to 1ml.
[0154] Preparation method:
[0155] (a) take by weighing the active ingredient and solid excipient of prescription quantity, add appropriate amount (65% of prescription full amount) water for injection, stir and make to dissolve,
[0156] (b) add activated carbon (0.1%) to the medicinal solution obtained in the previous step, stir (make adsorption for 15min), filter and decarbonize (after decarburization and filtration with a titanium rod with a pore size of 1um, then use a 0.45um polyethersulfone filter element to filter the liquid medicine coarse filtration);
[0157] (c) Add water for injection to the full amount of the prescription, stir eve...
Embodiment 2
[0161] Embodiment 2: prepare terazosin freeze-dried powder injection
[0162] formula:
[0163] Terazosin: 1mg,
[0164] Mannitol: 25mg,
[0165] Acid-base regulator: use appropriate amount when necessary, and adjust the pH to the specified value in the following preparation method,
[0166] Water for injection: up to 2.5ml.
[0167] Preparation method:
[0168] (a) Take by weighing the active ingredient and the solid auxiliary material of the prescription amount, add an appropriate amount (70% of the total amount of the prescription) water for injection, stir to dissolve,
[0169] (b) add activated carbon (0.15%) to the medicinal solution obtained in the previous step, stir (make adsorption for 10min), filter and decarbonize (after decarbonizing and filtering with a titanium rod with a pore size of 1um, and then use a 0.45um polyethersulfone filter element to filter the liquid medicine coarse filtration);
[0170] (c) Add water for injection to the full amount of ...
Embodiment 3
[0173] Embodiment 3: prepare terazosin freeze-dried powder injection
[0174] formula:
[0175] Terazosin: 1mg,
[0176] Mannitol: 500mg,
[0177] Acid-base regulator: use appropriate amount when necessary, and adjust the pH to the specified value in the following preparation method,
[0178] Water for injection: up to 5ml.
[0179] Preparation method:
[0180] (a) take by weighing the active ingredient and solid excipient of prescription quantity, add appropriate amount (60% of prescription full amount) water for injection, stir and make to dissolve,
[0181] (b) Add gac (0.05%) to the medicinal solution obtained in the previous step, stir (make adsorption for 20min), filter and decarbonize (after decarburization and filtration with a titanium rod with a pore size of 1um, then use a 0.45um polyethersulfone filter element to filter the liquid medicine coarse filtration);
[0182] (c) Add water for injection to the full amount of the prescription, stir evenly, measu...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com