L-histidine production method and special recombinant bacteria
一种重组菌、组氨酸的技术,应用在氨基酸发酵领域,能够解决菌株生长和葡萄糖代谢能力下降、菌株葡萄糖代谢能力减弱、L-组氨酸产量下降等问题,达到便于工业化控制、易于过程和成本控制、发酵周期短的效果
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0062] Embodiment 1, the acquisition of L-histidine chassis engineering bacteria CG160
[0063] 1. The promoter of the L-histidine synthesis operon in the wild-type Corynebacterium glutamicum ATCC13032 was replaced by a strong promoter P glyA
[0064] According to the hisEG operon of Corynebacterium glutamicum ATCC13032 in Genbank and its upstream and downstream sequences and P glyA The primers were designed separately for the promoter sequence.
[0065] Using Corynebacterium glutamicum ATCC13032 genomic DNA as a template, using P1 and P2 as primers to PCR amplify the upstream homology arm of the hisEG operon promoter; using P3 and P4 as primers to amplify the promoter P glyA ; Use P5 and P6 as primers to amplify the homology arm downstream of the hisEG promoter. Then use the purified PCR product as a template, use P1 and P6 as primers, and use overlap extension PCR technology (SOE) to amplify to obtain a PCR product of 1920bp, which is P glyA and replaced promoter P hisE...
Embodiment 2
[0123] Embodiment 2, the construction of the recombinant bacterium CG171 containing the high-production L-histidine of plasmid
[0124] 1. Construction of L-histidine primary engineering bacteria CG176
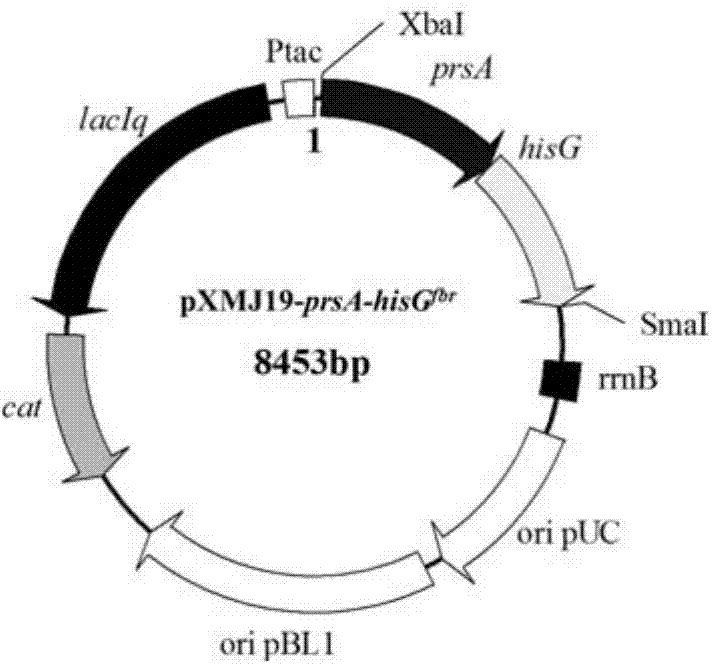
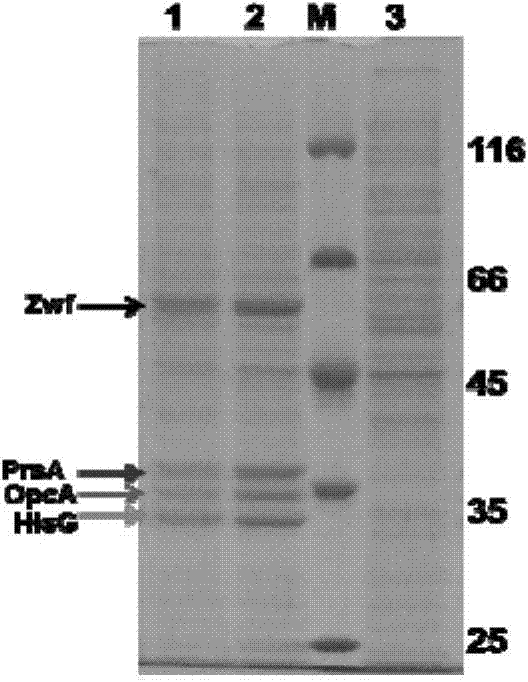
[0125] The genomic DNA of strain CG160 was used as a template, and the prsA gene (992bp) and hisG were amplified by PCR using P28 / P29 and P30 / P31 as primers, respectively fbr (860bp). The two genes were joined using overlap extension PCR to amplify hisG fbr and the prsA gene as a template, using P28 and P31 as primers for PCR amplification, and the PCR product of 1852bp is prsA-hisG fbr fragment (sequence 3).
[0126] Wherein, the nucleotides 1-992 from the 5' end of sequence 3 are prsA, and the nucleotides 993-1852 from the 5' end of sequence 3 are hisG fbr (hisG gene with three point mutations).
[0127] After the above PCR product was double-digested with Xba I and Sma I, it was ligated with the Corynebacterium glutamicum-Escherichia coli shuttle expression plasmid pXM...
Embodiment 3
[0165] Example 3, Construction of plasmid-free L-histidine high-yielding recombinant strain CG324
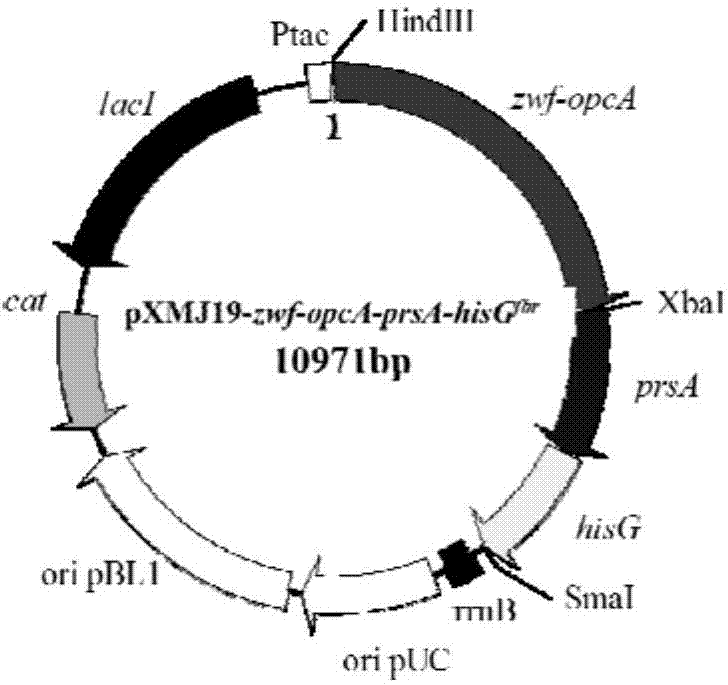
[0166] According to the prsA gene of Corynebacterium glutamicum ATCC13032 in Genbank and its upstream and downstream sequences and P sod The primers were designed separately for the promoter sequence.
[0167] Using the genomic DNA of Corynebacterium glutamicum ATCC13032 as a template, the upstream homology arm of the prsA promoter was amplified with P48 and P49 as primers; the prsA promoter was amplified with P50 and P51 as primers sod Promoter; use P52 and P53 as primers to amplify the downstream homology arm of prsA promoter. Then, using the purified PCR product as a template, using P48 and P53 as primers, and using overlap extension PCR (SOE) to amplify, a 1455bp PCR product was obtained, which was a P sod and replaced promoter P prsA A fragment of the upstream and downstream homology arms of (SEQ ID NO: 9).
[0168] Wherein, the 1-655th nucleotide from the 5' end of seq...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com