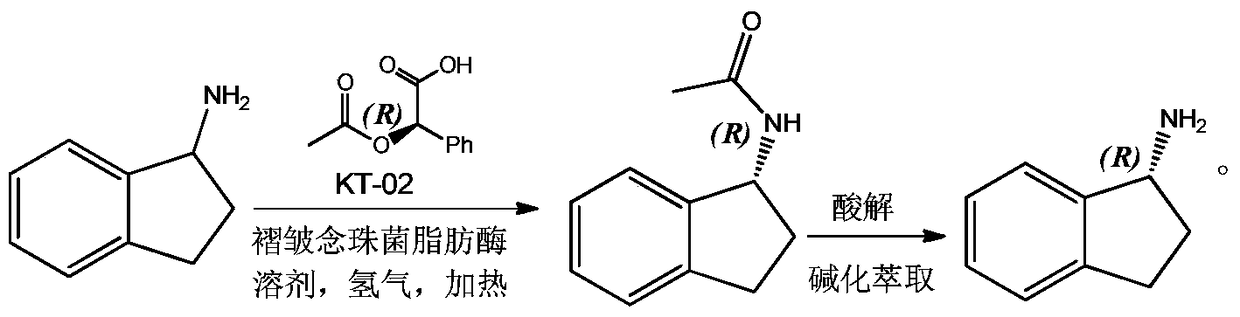
A kind of preparation method of r-1-aminoindane
An aminoindan and acyl technology, which is applied in the field of separation and preparation of optically pure chiral compounds, can solve the problems of low corresponding selectivity, high cost, low utilization rate of raw materials, etc., and achieves high optical purity, stable properties, and utilization of raw materials. full effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0007] 1. Resolution of 1-aminoindane
[0008] In a 1000ML autoclave, add 500ML toluene, 66.6G 1-aminoindan, 115.9g D-(-)-O-acetylmandelic acid, 4g Candida plicata lipase and 6g KT-02, seal the autoclave, and blow it with nitrogen After replacing the air in the autoclave, feed hydrogen into the autoclave to a pressure of 1.0MP, start stirring, and raise the temperature to 50°C for reaction; after 18 hours, take a sample and detect that 1-aminoindane is completely converted into R-1-amino Acetyl compound of indane; after the reaction, the solution was concentrated and subjected to column chromatography to obtain 84.1 g of acetyl compound of pure R-1-aminoindane with a yield of 96.1%.
[0009] 2. Acid hydrolysis to obtain R-1-aminoindan salt
[0010] Take several times to get 87.6g of the acetyl compound of R-1-aminoindane prepared in the previous step and add it to 1000ml of ethanol and concentrated hydrochloric acid in a volume ratio of 1:1, then heat to reflux and react for ...
Embodiment 2
[0014] 1. Resolution of 1-aminoindane
[0015] In a 1000ML autoclave, add 500ML toluene, 66.6G1-aminoindan, 144.9g D-(-)-O-acetylmandelic acid, 5g Candida plicata lipase and 10g KT-02 successively, seal the autoclave, and use Nitrogen replaced the air in the autoclave, then introduced hydrogen into the autoclave to a pressure of 1.5MP, started stirring, and raised the temperature to 70°C for reaction; after 14 hours, sampling and testing showed that 1-aminoindane was completely converted into R-1 - the acetyl compound of aminoindane; after the reaction, the solution was concentrated and subjected to column chromatography to obtain 82.9 g of the acetyl compound of R-1-aminoindane, with a yield of 94.7%.
[0016] 2. Acid hydrolysis to obtain R-1-aminoindan salt
[0017] Take 87.6g of the acetyl compound of R-1-aminoindane obtained by repeating the previous step several times and add it to the solution mixed with 1000ml of ethanol and concentrated sulfuric acid at a volume ratio...
PUM
| Property | Measurement | Unit |
|---|---|---|
| optical purity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to view more
Login to view more - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap