Internet medicine quality credit assessment method and system
A drug quality and credit evaluation technology, applied in data processing applications, commerce, instruments, etc., can solve the problems of drug quality credit evaluation, abstraction, and information asymmetry, to ensure drug quality and safety, and solve drug quality information inconsistencies symmetrical effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
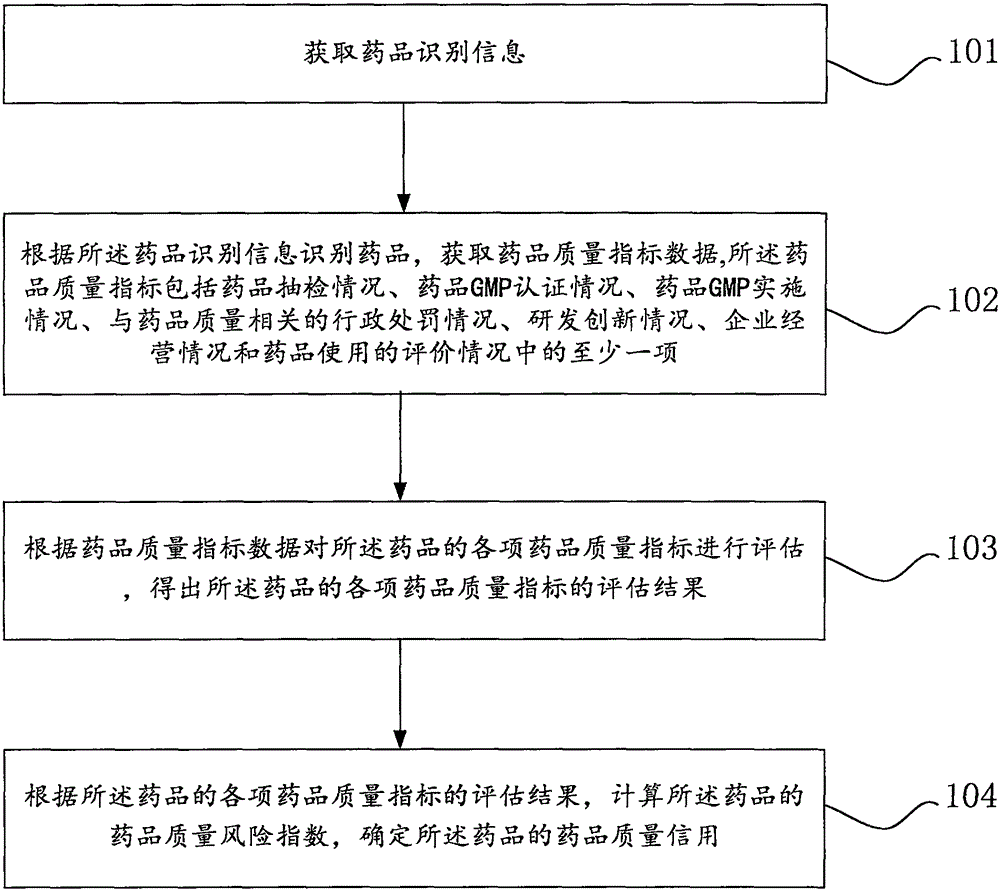
[0052] Such as figure 1 As shown, the Internet drug quality credit evaluation method of the embodiment of the present invention, the Internet drug quality credit evaluation method includes:
[0053] Step 101: Obtain drug identification information.
[0054] Wherein, the acquiring drug identification information may include: receiving drug identification information input by the user; and / or, scanning the drug identification code to obtain the drug identification information; and / or, receiving the drug identification information imported from the database file and / or, receiving Drug identification information captured from Internet pages.
[0055] In this embodiment, the user can input the drug identification information that needs to be evaluated for drug quality credit in different forms, and then obtain the relevant data of various drug quality indicators of the drug, and the drug quality index data is comprehensive and accurate.
[0056]Step 102: Identify the drug accordi...
Embodiment 2
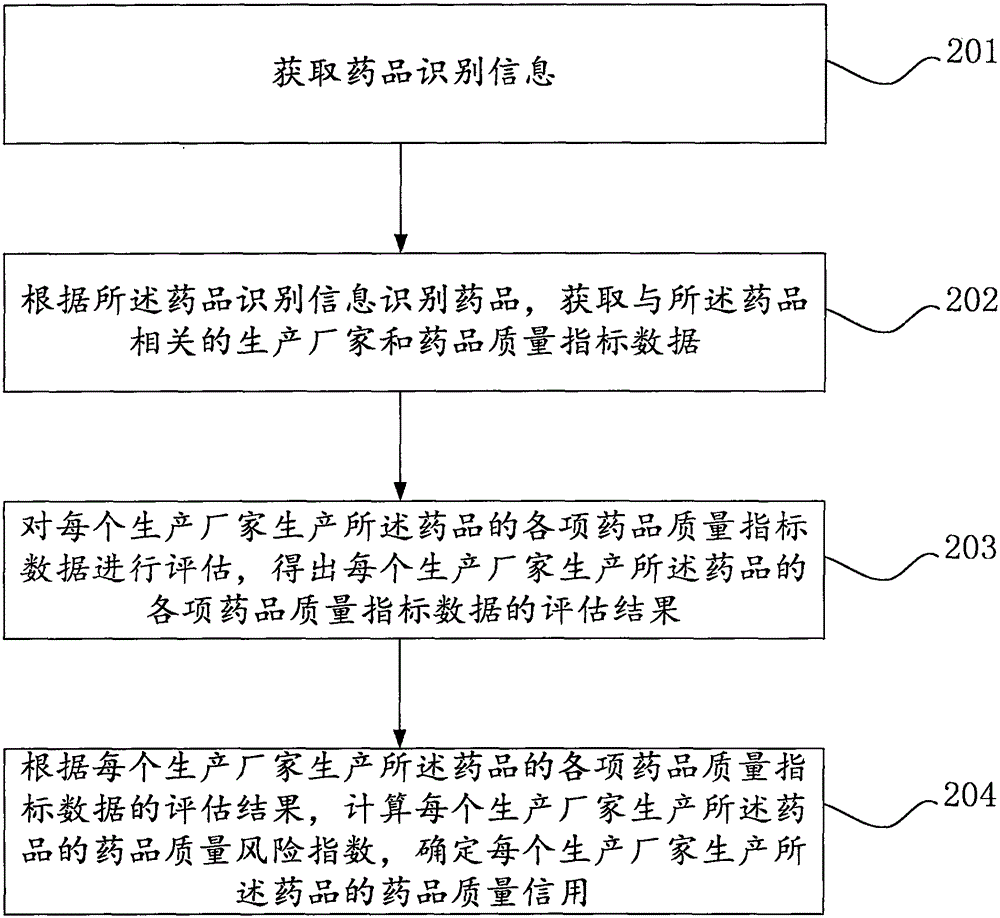
[0083] Specifically, such as figure 2 as shown,
[0084] Step 201: Obtain drug identification information.
[0085] Step 202: Identify the drug according to the drug identification information, and obtain the manufacturer and drug quality index data related to the drug. The drug quality index includes drug sampling inspection status, drug GMP certification status, drug GMP implementation status, and drug quality index data. At least one of the relevant administrative punishment situation, research and development innovation situation, business operation situation and drug use evaluation situation.
[0086] Step 203: Evaluate the various drug quality index data of the drug produced by each manufacturer, and obtain the evaluation results of each drug quality index data of the drug produced by each manufacturer.
[0087] Wherein, the evaluation results of each drug quality index data of the drug produced by each manufacturer are evaluated to obtain the evaluation results of ea...
Embodiment 3
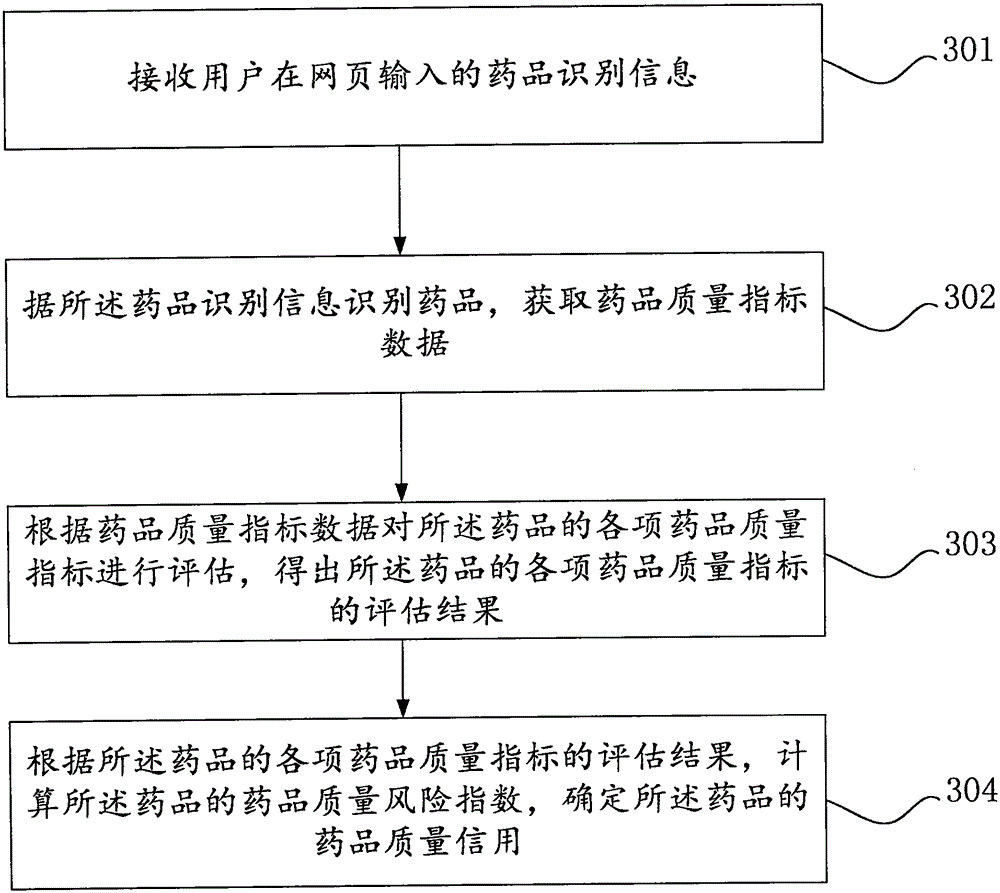
[0116] Such as image 3 As shown, the Internet drug quality credit evaluation method of the embodiment of the present invention, the Internet drug quality credit evaluation method includes:
[0117] Step 301: Receive the drug identification information input by the user on the webpage.
[0118] Among them, the drug identification information entered by the user on the web page can be the generic name of the drug and the drug manufacturer input at the same time, the generic name of the drug, the manufacturer and the specification of the drug input at the same time, the trade name and specification of the drug input at the same time, the trade name of the drug entered separately or Drug approval number entered separately.
[0119] Step 302: Identify the drug according to the drug identification information, and obtain drug quality index data. The drug quality index includes drug sampling inspection, drug GMP certification, drug GMP implementation, drug quality-related administr...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com