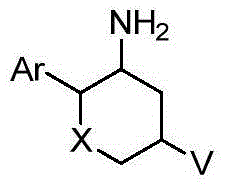
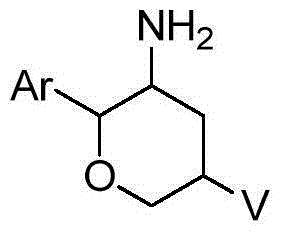
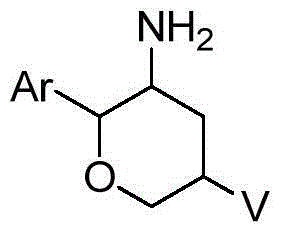
Fused tricyclic substituted amino six-membered ring derivative and application thereof in medicine
An amino six-membered ring type, fused ring technology, applied in the field of dipeptidyl peptidase IV inhibitors, can solve problems such as diabetes burden
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0229] 2-(5-((3R,5S,6R)-5-amino-6-(2,5-difluorophenyl)tetrahydro-2H-pyran-3-yl)-3b,4,5,6 ,6a,7-hexahydro-2H-pyrrolo[3',4':3,4]cyclopento[1,2-c]pyrazol-2-yl)ethanol (Compound 1-1)
[0230] 2-(5-((3R,5S,6R)-5-amino-6-(2,5-difluorophenyl)tetrahydro-2H-pyran-3-yl)-3b,4,5,6,6a,7- hexahydro-2H-pyrrolo[3',4':3,4]cyclopenta[1,2-c]pyrazol-2-yl)ethanol
[0231]
[0232] 2-(5-((3R,5S,6R)-5-amino-6-(2,5-difluorophenyl)tetrahydro-2H-pyran-3-yl)-3b,4,5,6 ,6a,7-Hexahydro-1H-pyrrolo[3',4':3,4]cyclopento[1,2-c]pyrazol-1-yl)ethanol (Compound 1-2)
[0233] 2-(5-((3R,5S,6R)-5-amino-6-(2,5-difluorophenyl)tetrahydro-2H-pyran-3-yl)-3b,4,5,6,6a,7- hexahydro-1H-pyrrolo[3',4':3,4]cyclopenta[1,2-c]pyrazol-1-yl)ethanol
[0234]
[0235]
[0236] The first step: tert-butyl 2-(2-ethoxy-2-carbonylethyl)-3b,4,6a,7-tetrahydro-2H-pyrrolo[3',4':3,4] ring Pento[1,2-c]pyrazole-5(6H)-carboxylate (1a-1)
[0237] tert-butyl2-(2-ethoxy-2-oxoethyl)-3b,4,6a,7-tetrahydro-2H-pyrrolo[3',4':3,4]cyclopenta
...
Embodiment 2
[0274] (2R,3S,5R)-2-(2,5-difluorophenyl)-5-(3b,4,6a,7-tetrahydro-2H-pyrrolo[3',4':3,4] Cyclopenta[1,2-c]pyrazol-5(6H)-yl)tetrahydro-2H-pyran-3-amino (compound 2)
[0275] (2R,3S,5R)-2-(2,5-difluorophenyl)-5-(3b,4,6a,7-tetrahydro-2H-pyrrolo[3',4':3,4]cyclopenta[1,2 -c]pyrazol-5(6H)-yl)tetrahydro-2H-pyran-3-amine
[0276]
[0277] The first step: (3b,4,5,6,6a,7-hexahydro-2H-pyrrolo[3',4':3,4]cyclopento[1,2-c]pyrazolebenzenesulfonic acid salt (2a)
[0278] 3b,4,5,6,6a,7-hexahydro-2H-pyrrolo[3',4':3,4]cyclopenta[1,2-c]pyrazolebenzenesulfonate
[0279] At room temperature, intermediate 2 (0.250 g, 1.00 mmol) and benzenesulfonic acid (0.250 g, 1.50 mmol) were dissolved in methanol (6 mL) and reacted at 68° C. for 12 hours. The reaction solution was concentrated under reduced pressure to obtain a white solid 2a (0.362 g, yield 100%).
[0280] MS m / z (ESI): 150.3 [M+1].
[0281]The second step: tert-butyl ((2R,3S,5R)-2-(2,5-difluorophenyl)-5-(3b,4,6a,7-tetrahydro-2H-pyrrolo[3...
Embodiment 3
[0290] Example 3 Two optical isomers of compound 2, namely compound 3-1 and compound 3-2
[0291] Using intermediate 2-1 as raw material, compound 3-1 was obtained with reference to the synthetic method of Example 2;
[0292] MSm / z(ESI): 361.3[M+1];
[0293] 1 HNMR (400MHz, CDCl 3 ):δ7.21(s,1H),δ7.14-6.99(m,3H),4.18-4.16(d,1H),4.14-4.09(m,1H),3.52-3.50(m,1H),3.43 -3.29(d,1H), 2.97-2.91(m,3H), 2.78-2.53(m,1H), 2.44-2.31(m,5H), 1.40-1.13(m,4H).
[0294] Using intermediate 2-2 as raw material, compound 3-2 was obtained with reference to the synthetic method of Example 2;
[0295] MSm / z(ESI): 361.3[M+1];
[0296] 1 HNMR (400MHz, CDCl 3 ):δ7.17(s,1H),7.10-6.95(m,3H),4.14-4.12(d,1H),4.10-4.02(m,1H),3.52-3.43(m,1H),3.43(m , 1H), 3.43-3.29(d, 1H), 2.97-2.91(m, 2H), 2.78-2.53(m, 1H), 2.71-2.36(m, 5H), 1.40-1.13(m, 4H).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com