A kind of ileximide sustained-release preparation and preparation method thereof
A sustained-release preparation, hydroxypropyl methylcellulose technology, applied in the field of eleximide sustained-release preparations and its preparation, can solve the problems of poor patient compliance, inconvenient taking, and large fluctuations in blood drug concentration of ordinary tablets. To achieve the effect of reducing toxic and side effects, increasing compliance and increasing curative effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
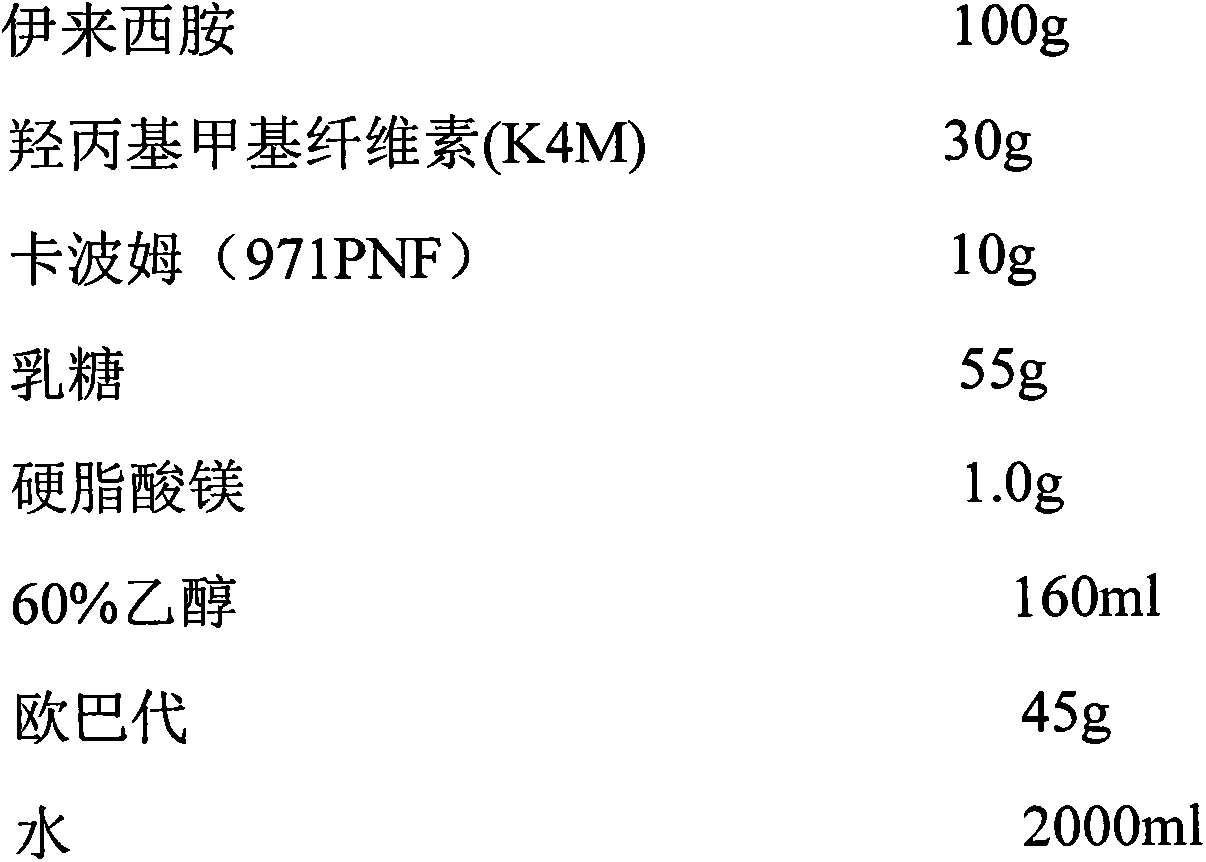
[0027] Prescription (1000 tablets):
[0028]
[0029]
[0030] Preparation Process:
[0031] 1) Crush all raw and auxiliary materials through a 60-80 mesh sieve
[0032] 2) Mix the ilexamine, sustained-release materials, regulated release materials, and filling materials evenly;
[0033] 3) Add binder to make soft material, granulate and dry;
[0034] 4) After sizing the dried granules, it is preferable to add lubricant, mix well, and compress;
[0035] 5) Then wrap it with a moisture-proof film coating, the coating will increase the weight by 2-3%, and it will be dried.
Embodiment 2
[0037] Prescription (1000 tablets):
[0038]
[0039] Preparation Process:
[0040] 1) Crush all raw and auxiliary materials through a 60-80 mesh sieve
[0041] 2) Mix the ilexamine, sustained-release materials, regulated release materials, and filling materials evenly;
[0042] 3) Add binder to make soft material, granulate and dry;
[0043] 4) After sizing the dried granules, it is preferable to add lubricant, mix well, and compress;
[0044] 5) Then wrap it with a moisture-proof film coating, the coating will increase the weight by 2-3%, and it will be dried.
Embodiment 3
[0046] Prescription (1000 tablets):
[0047]
[0048] Preparation Process:
[0049] 1) Crush all raw and auxiliary materials through a 60-80 mesh sieve
[0050] 2) Mix the ilexamine, sustained-release materials, regulated release materials, and filling materials evenly;
[0051] 3) Add binder to make soft material, granulate and dry;
[0052] 4) After sizing the dried granules, it is preferable to add lubricant, mix well, and compress;
[0053] 5) Then wrap it with a moisture-proof film coating, the coating will increase the weight by 2-3%, and it will be dried.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com