Application of zoledronic acid in preparation of drugs for treating fatty liver diseases
A technology of fatty liver disease and zoledronic acid, which is applied in the field of zoledronic acid for the preparation of drugs for the treatment of fatty liver disease, can solve the problems that there is no zoledronic acid, etc., achieve low development, slow down steatosis, slow down The effect of lipid accumulation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0034] Method 2 Preparation of Paraffin Sections
[0035] An appropriate amount of right lobe of liver tissue was taken, fixed with 4% paraformaldehyde for 24-48 hours, dehydrated with ethanol step by step, transparent in xylene, dipped in wax, embedded in paraffin, and sectioned with a thickness of 5 μm.
[0036] Method 3 HE staining of paraffin sections
[0037] The slices were soaked in xylene for 10min and 5min to dewax, and then hydrated in graded ethanol. After staining with hematoxylin for 4 minutes, wash with distilled water. with 0.25% NH 3 ·H 2After O bluish for 30s, wash with single distilled water. After dehydration with graded ethanol to 95% ethanol, stain with 0.5% eosin solution for 2-3 seconds, then dehydrate in 100% ethanol, and soak in xylene for 2 minutes. Finally, the slides were sealed with neutral gum.
[0038] Method 4 Preparation of Frozen Sections
[0039] An appropriate amount of tissue was taken from the same part of the right lobe of the live...
Embodiment 1
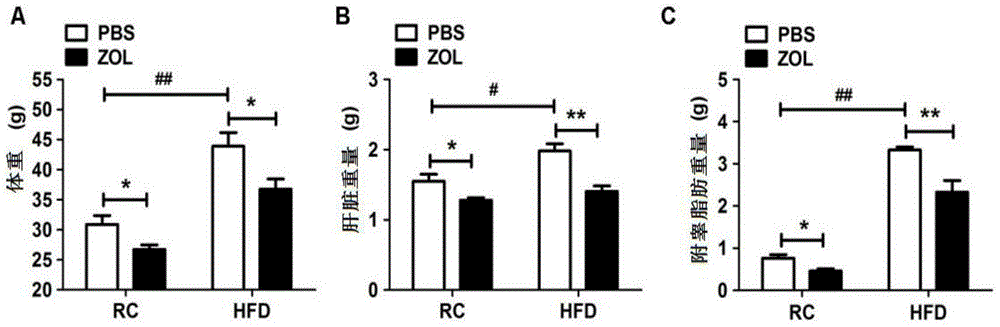
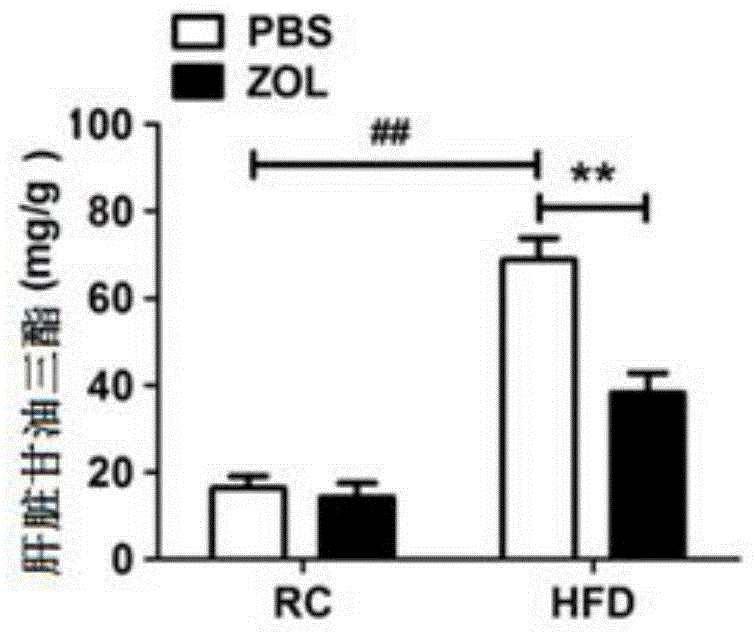
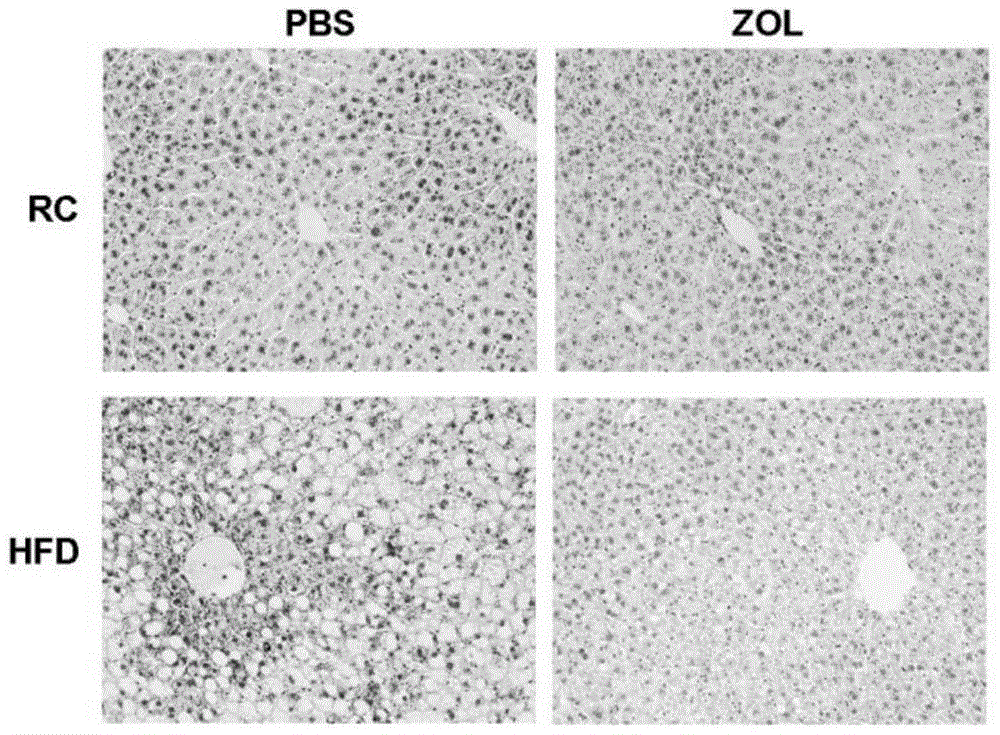
[0052] Example 1 Effect of zoledronic acid on food-induced non-alcoholic fatty liver
[0053] Clinically, fatty liver occurs in patients, and the liver is swollen in appearance, but the main reason is due to the accumulation of lipids. And this kind of lipid accumulation can be obtained by biopsy to obtain liver tissue, and the accumulation of lipid can be seen through HE staining and oil red staining, so direct detection of HE and oil red is a better way to observe lipid accumulation.
[0054] According to the aforementioned general materials and methods, firstly, zoledronic acid and placebo were administered to the mice in the high-fat diet group. In this embodiment, zoledronic acid is mainly administered through tail vein injection, and the main action site of the drug is the liver, and is injected once every two days. The dosage was 50 μg / kg zoledronic acid. Tissue triglyceride level detection and HE staining were performed on mice after administration, the experimental ...
Embodiment 2
[0061] Example 2 Effect of zoledronic acid on food-induced fatty liver injury
[0062] Similar to Example 1, according to the foregoing general materials and methods, at first high-fat-induced mice were administered with zoledronic acid and a placebo, and alanine aminotransferase (ALT) and aspartate aminotransferase (AST) in mouse blood were detected after administration. ) level, the experimental data are shown in Table 3, and the results are as follows Figure 4 A and 4B are shown.
[0063] The levels of alanine aminotransferase (ALT) and aspartate aminotransferase (AST) in mouse blood after the administration of table 3
[0064]
[0065]
[0066] The HFD group was a high-fat diet group, the RC group was a normal diet group, PBS was a placebo, ZOL was zoledronic acid, and the dosage was 50 μg / kg.
[0067] It can be seen from Table 3 that for the HFD group, the levels of ALT and AST decreased significantly after administration of ZOL. Among them, in RC group, ALT and...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com