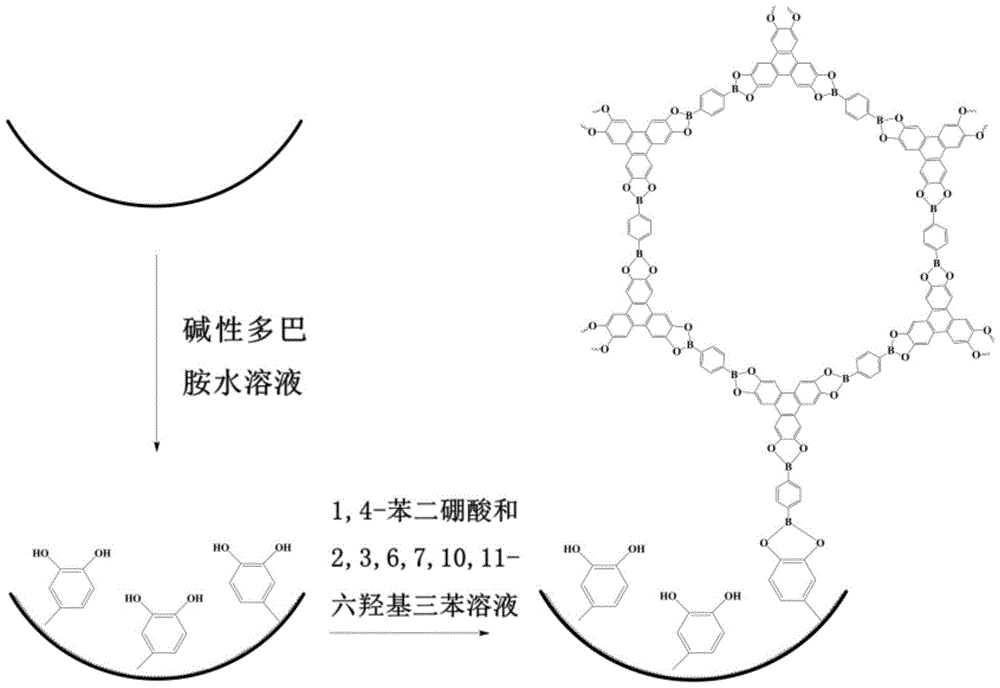
A method for immobilizing covalent organic framework materials and its application
A covalent organic framework and coating technology, applied in the field of chromatographic columns, achieves the effects of less sample consumption, simple process and low cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
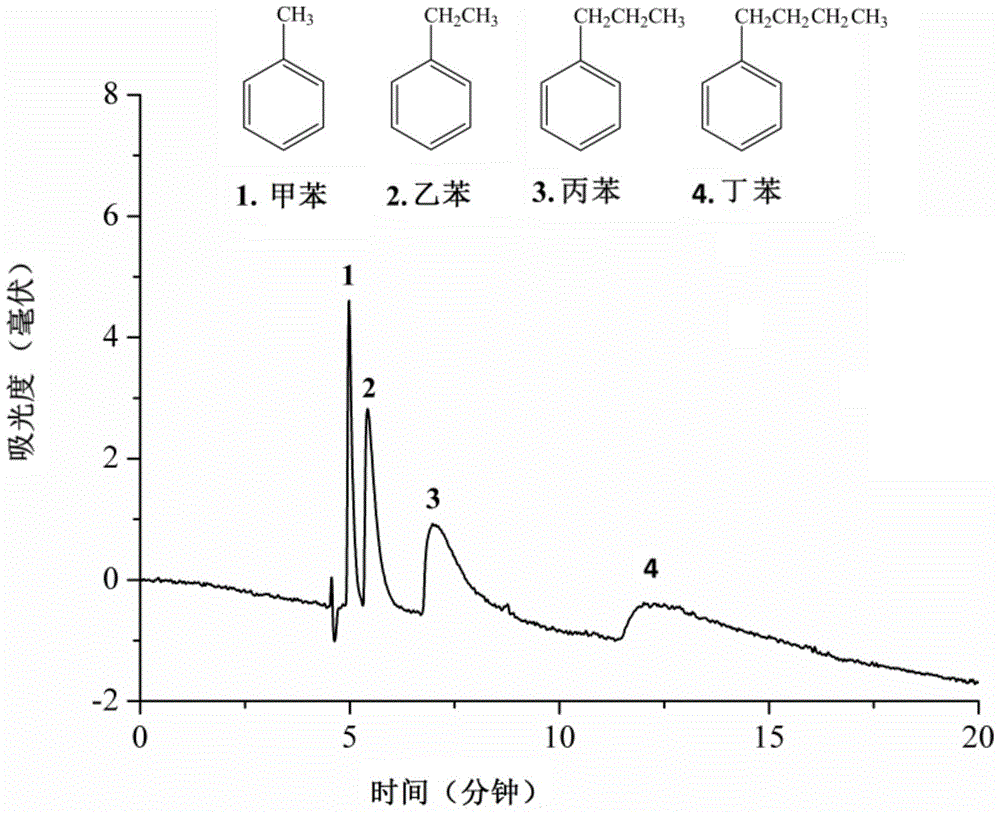
Image
Examples
Embodiment 1
[0029] A 31 cm long quartz capillary (50 μm i.d.×375 μm o.d.) was rinsed with methanol for 2 h and dried with nitrogen; at room temperature, the pre-oxidized dopamine aqueous solution (concentration 2 mg / mL, pH 8.5) was continuously passed into the quartz capillary for 10 h, Then rinse with pure water and dry with nitrogen to obtain a capillary with a uniform and stable polydopamine coating on the inner wall; Trimethylbenzene / 1,4-dioxane (v / v, 1:1) mixed reaction solution (concentrations of 1,4-benzenediboronic acid and 2,3,6,7,10,11-hexahydroxytriphenyl 0.75mM, 0.5mM respectively) into the quartz capillary with polydopamine coating on the inner wall for 5min, then seal both ends of the quartz capillary, place it in an ultrasonic instrument for ultrasonic treatment for 1h, heat the reaction in an oil bath at 100°C for 20h, and rinse with methanol for 8h , blown dry with nitrogen to obtain a quartz capillary with a COF-5 coating on the inner wall.
Embodiment 2
[0031] Rinse a 60cm-long polyetheretherketone capillary (PEEK tube) (50μm i.d.×360μm o.d.) with methanol for 2h, and dry it with nitrogen; Continue to pass through the PEEK tube for 8 hours, rinse with pure water, and blow dry with nitrogen to obtain a PEEK tube with a uniform and stable polydopamine coating on the inner wall; mix 1,4-benzenediboronic acid and 2,3,6,7,10,11 -Mesitylene / 1,4-dioxane (v / v, 1:1) mixed reaction solution of hexahydroxytriphenyl (1,4-benzenediboronic acid and 2,3,6,7,10,11 -The concentration of hexahydroxytriphenyl is 0.75mM, 0.75mM respectively) into the PEEK tube with polydopamine coating on the inner wall for 3min, then seal the two ends of the PEEK tube, place it in an ultrasonic instrument for ultrasonic treatment for 1.5h, and 110°C oil The reaction was heated in the bath for 30 hours, rinsed with methanol for 8 hours, and dried with nitrogen to obtain a PEEK tube with a COF-5 coating on the inner wall.
Embodiment 3
[0033] Rinse a 15cm-long polytetrafluoroethylene capillary (PTFE tube) (200μm i.d.×300μm o.d.) with methanol for 2h, and dry it with nitrogen; Continue to pass through the polytetrafluoroethylene capillary for 12 hours, rinse with pure water, and blow dry with nitrogen to obtain a polytetrafluoroethylene capillary with a uniform and stable polydopamine coating on the inner wall; mix 1,4-benzenediboronic acid and 2,3, 6,7,10,11-hexahydroxytriphenyl mesitylene / 1,4-dioxane (v / v, 1:1) mixed reaction solution (1,4-benzenediboronic acid and 2,3, The concentration of 6,7,10,11-hexahydroxytriphenyl is 0.75mM, 0.25mM respectively) into the polytetrafluoroethylene capillary with polydopamine coating on the inner wall for 1min, and then the two ends of the polytetrafluoroethylene capillary are sealed , placed in a sonicator for ultrasonic treatment for 2 hours, heated in an oil bath at 120°C for 10 hours, rinsed with methanol for 8 hours, and dried with nitrogen to obtain a polytetrafluo...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com