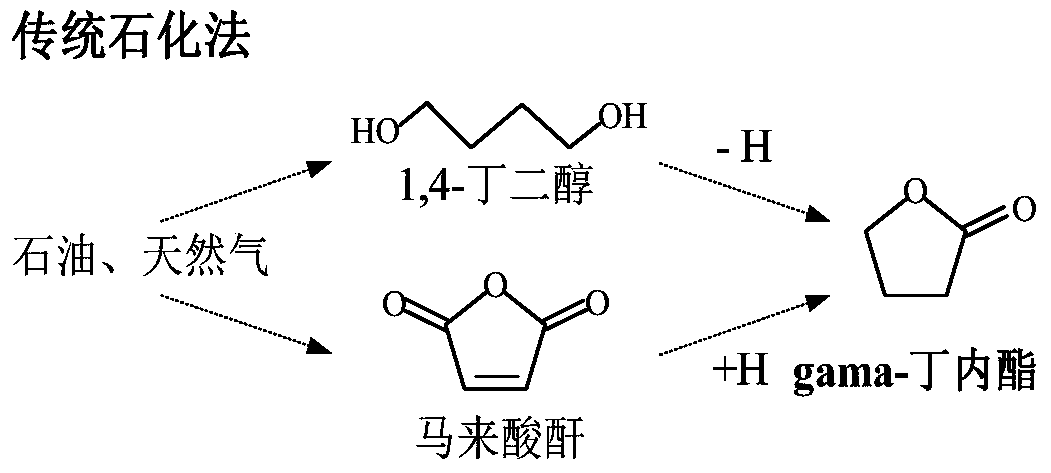
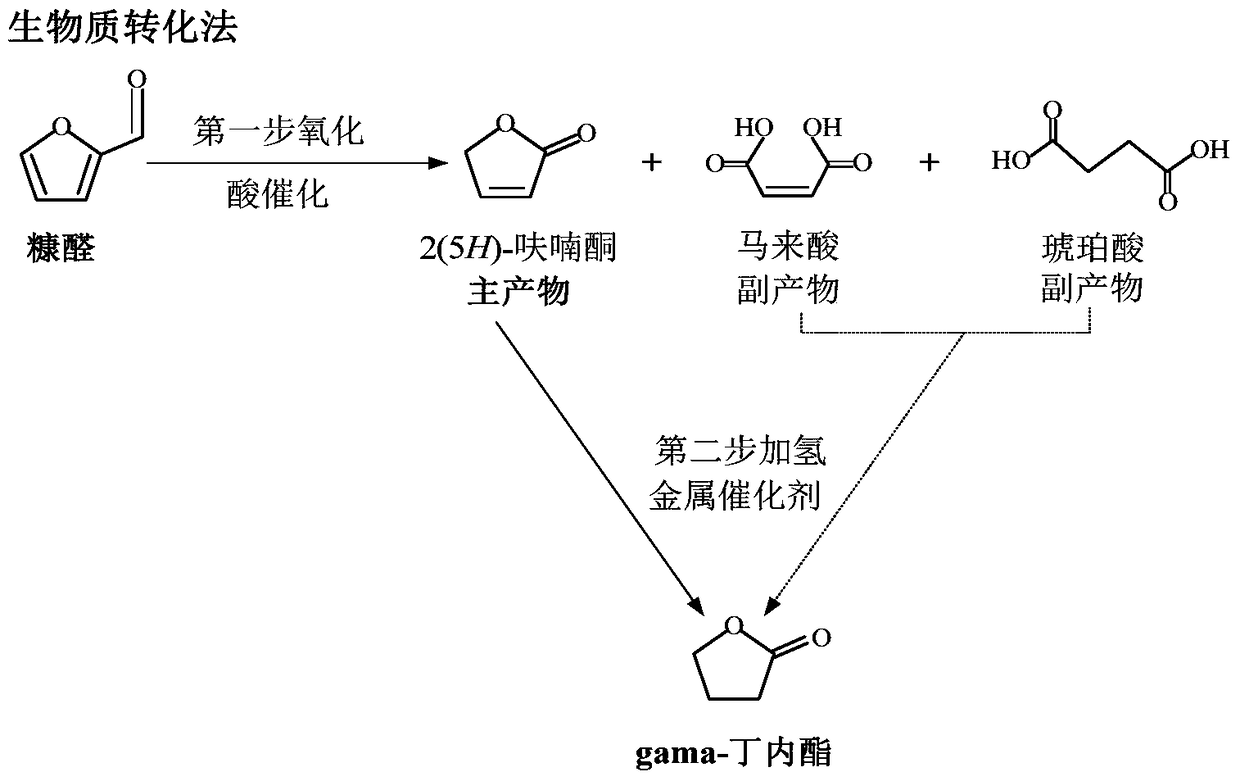
A kind of method using furfural as raw material to prepare gamma-butyrolactone
A technology of butyrolactone and furfural, which is applied in the field of preparation of γ-butyrolactone, can solve the problems of low selectivity, overcapacity of furfural, slow reaction rate, etc., and achieve the effect of simple process flow, avoiding high pollution and mild operating conditions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0040] Furfural oxidation reaction: add 4.0g furfural, 4.0g anhydrous sodium sulfate, 1.5ml formic acid and 1.5ml deionized water to 10ml of 1,2-dichloroethane, inject 10ml of hydrogen peroxide (30%), at 60 The reaction was stirred under normal pressure at ℃ for 2.5 hours. After the reaction, the mixture was allowed to stand for stratification, and the organic phase was distilled under reduced pressure to obtain 60% yield of 2(5H)-furanone and 18% yield of dibasic acid in the aqueous phase.
Embodiment 2
[0042] Furfural oxidation reaction: add 4.0g furfural, 4.0g anhydrous sodium sulfate, 1.5ml formic acid and 1.5ml deionized water to 10ml ethyl acetate, inject 10ml hydrogen peroxide (30%), stir at 60°C under normal pressure The reaction was carried out for 2.5 hours. After the reaction, the mixture was left to stand and separated, and the organic phase was distilled under reduced pressure to obtain 62% yield of 2(5H)-furanone and 15% yield of dibasic acid in the aqueous phase.
Embodiment 3
[0044] Furfural oxidation: add 4.0g furfural, 4.0g anhydrous sodium sulfate, and 1.5ml formic acid to 10ml methanol, inject 10ml hydrogen peroxide (30%), stir and react at 60°C for 2.5 hours under normal pressure, 2(5H)- The yield of furanone is 13%, and the yield of dibasic acid is 48%.
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com