Stapling eif4e interacting peptides
An unnatural amino acid, free technology, applied in the field of molecular biology
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment approach
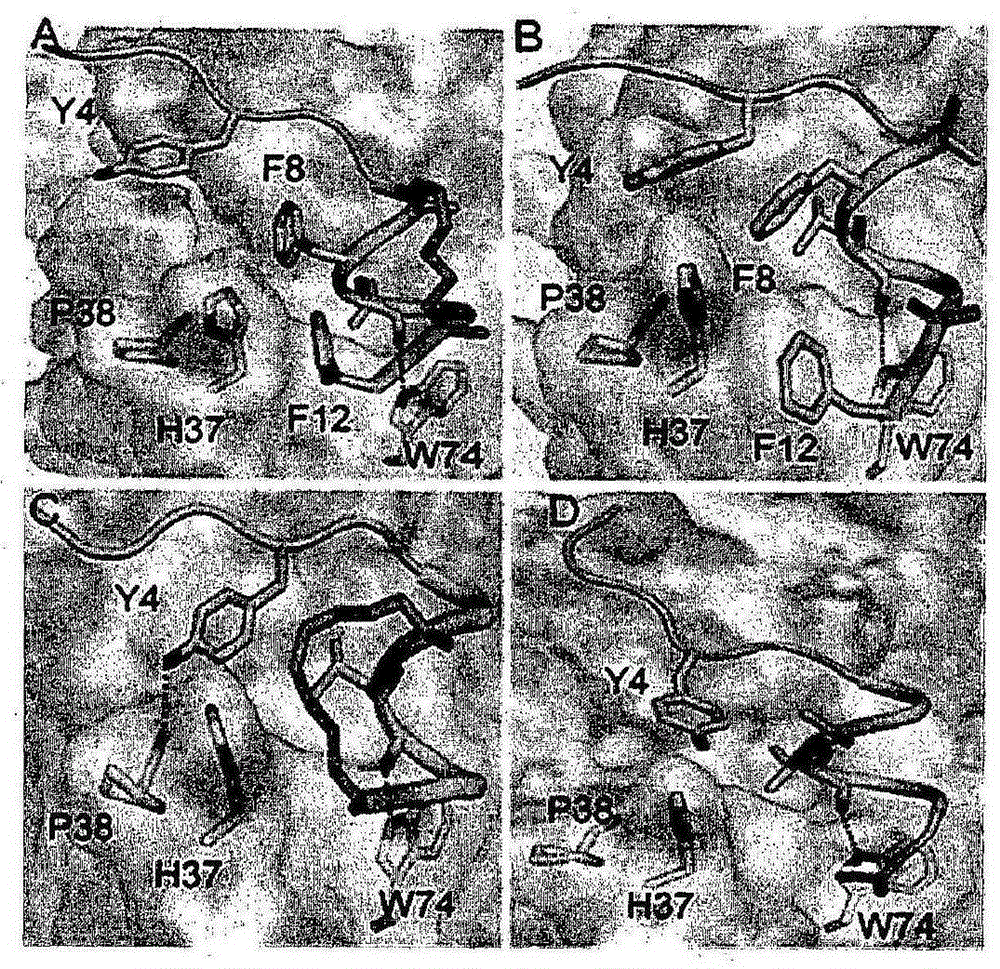
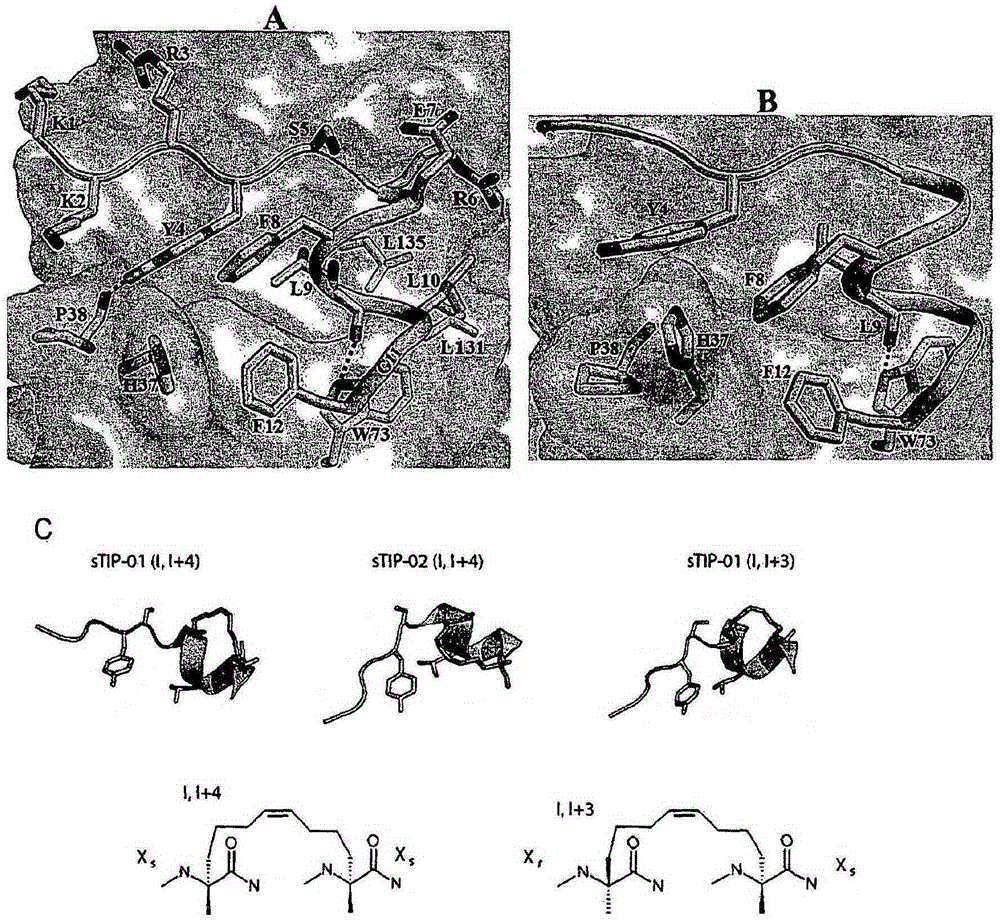
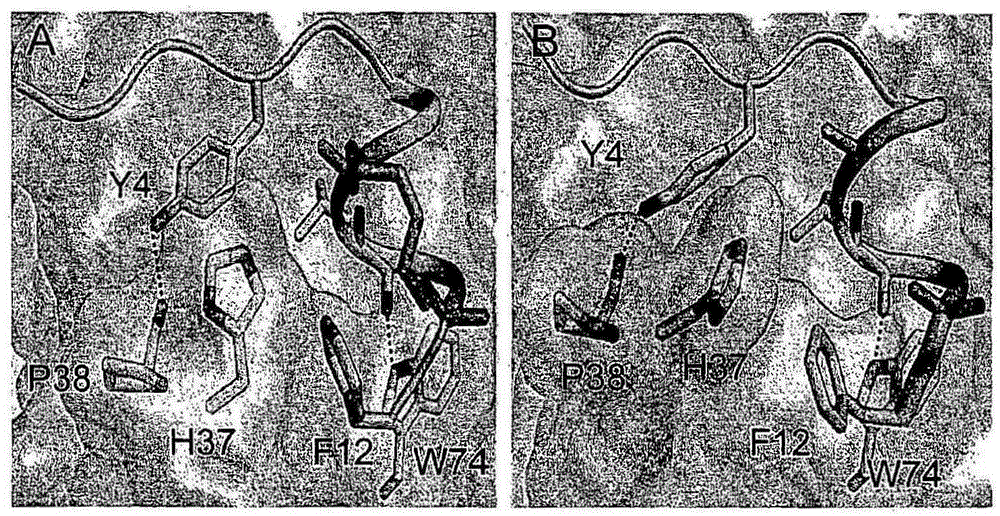
[0170] Inhibition of the protein interface between eIF4E and eIF4G is attractive for designing anticancer therapeutics. Peptides derived from eIF4G1 and 4EBP1 (inhibitors of eIF4E:eIF4G interaction) containing residues responsible for their interaction with eIF4E (YXXXXLΦ motif, where Φ denotes any hydrophobic residue) are structural mimics of each other. Tyrosine (Y4) ( image 3 ) is involved in various van der Waals contacts with eIF4E and the h-bond between its side chain hydroxyl and the carbonyl backbone of P38 of eIF4E. Leucine (L9) creates a shallow cavity on the surface of eIF4E and interacts with W73 of eIF4E through an h-bond between its backbone and the indole of tryptophan. A conserved hydrophobic residue (L10) squeezes L131 and L135 of eIF4E. The crystal structures of the two peptides in complex with eIF4E were approximately 50% α-helical; however, they contained negligible helical content in solution.
[0171] Therefore, the present inventors have improved sma...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com