2-Hydroxychalcone amine compound, its preparation method and use
A technology of hydroxychalcone amines and compounds, applied in the field of 2-hydroxychalcone amines, their preparation and application, capable of solving problems such as unsatisfactory depolymerization activity and poor curative effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
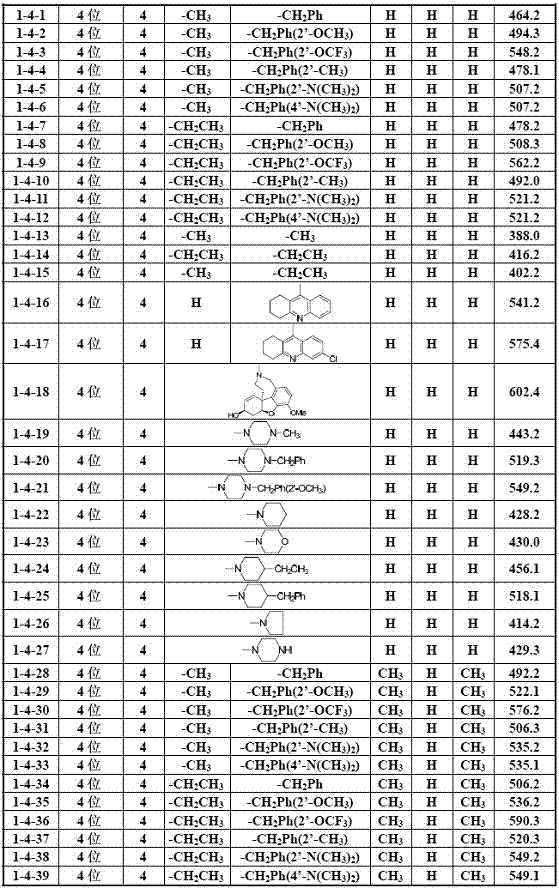
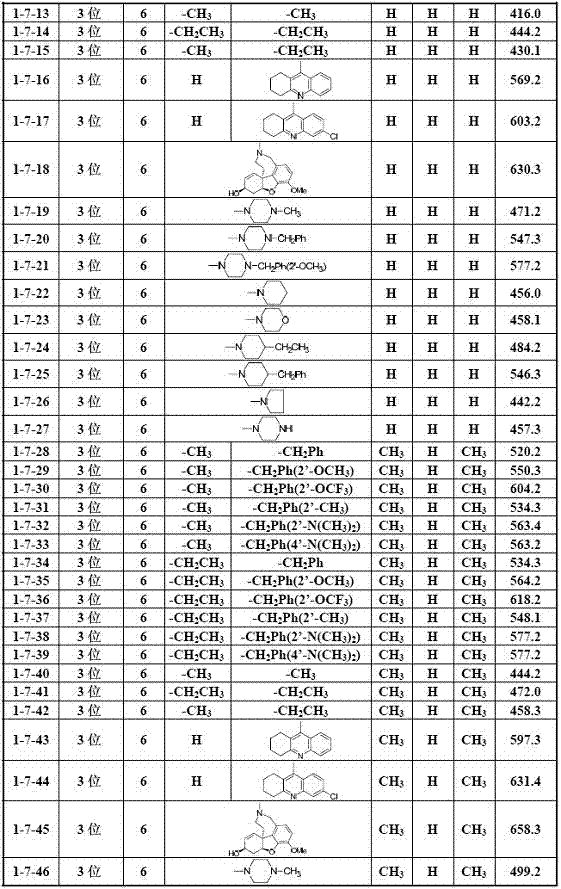
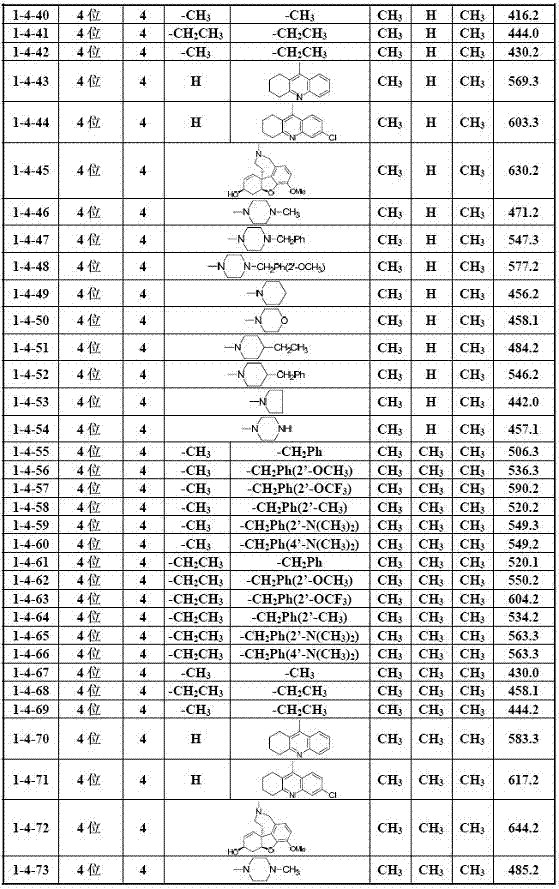
[0035] Example 1 General method for the preparation of 2-hydroxychalcone amines (I)
[0036] Add 2.0 mmol of the corresponding 2-hydroxyacetophenone compound (2), 3.0 mmol of the corresponding benzaldehyde compound (1) and 30 ml of ethanol into the reaction flask, stir well, then add 12.0 mmol of 30% KOH aqueous solution dropwise, Stir and react at 40-50°C for 2.0 to 72.0 hours (the reaction progress is tracked by TLC); after the reaction, cool to room temperature, adjust the pH of the reaction solution to strong acidity with 10% hydrochloric acid aqueous solution, and then adjust the pH of the reaction solution with saturated sodium bicarbonate aqueous solution To weak alkalinity, evaporate ethanol under reduced pressure, add 100 mL deionized water to the residual liquid, extract three times with 300 mL dichloromethane, combine organic layers, wash with saturated aqueous sodium chloride solution, and dry over anhydrous sodium sulfate After filtration, the solvent was evaporat...
Embodiment 2
[0061] Example 2 General method for the preparation of 2-hydroxychalcone amine compound (I) and acid salt formation
[0062] Add 2.0 mmol of 2-hydroxychalcone amine compound (I) obtained according to the above-mentioned Example 1 and 50 ml of acetone into the reaction flask, stir well, add 8.0 mmol of the corresponding acid, heat and reflux and stir for 20 minutes, and the reaction is over After cooling to room temperature, the solvent was evaporated under reduced pressure, the residue was recrystallized with acetone, and the precipitated solid was filtered to obtain the salt of 2-hydroxychalcone amine compound (I). 1 Confirmed by H NMR and ESI-MS.
Embodiment 3
[0063] Example 3 Part of the biological activity screening results of 2-hydroxychalcone amine compounds (I)
[0064] .
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com