Application of pentacyclic triterpene compound and medicine composition
A technology of pentacyclic triterpenoids and compounds, which is applied in the application of pentacyclic triterpenoids and the field of pharmaceutical compositions, can solve the problems of high toxicity and side effects, insufficient drug efficacy, poor bioavailability, etc., and achieve high inhibitory activity Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
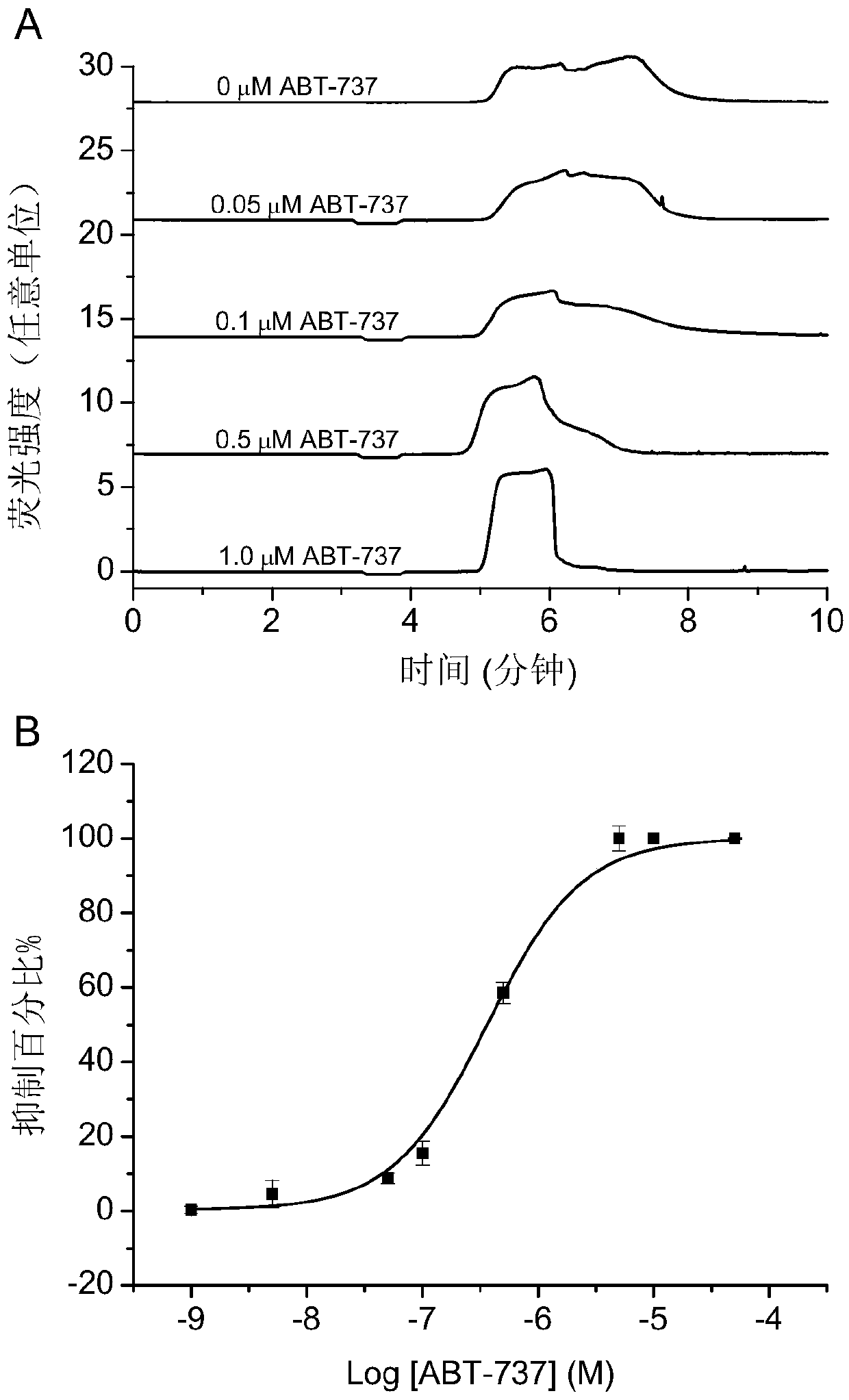
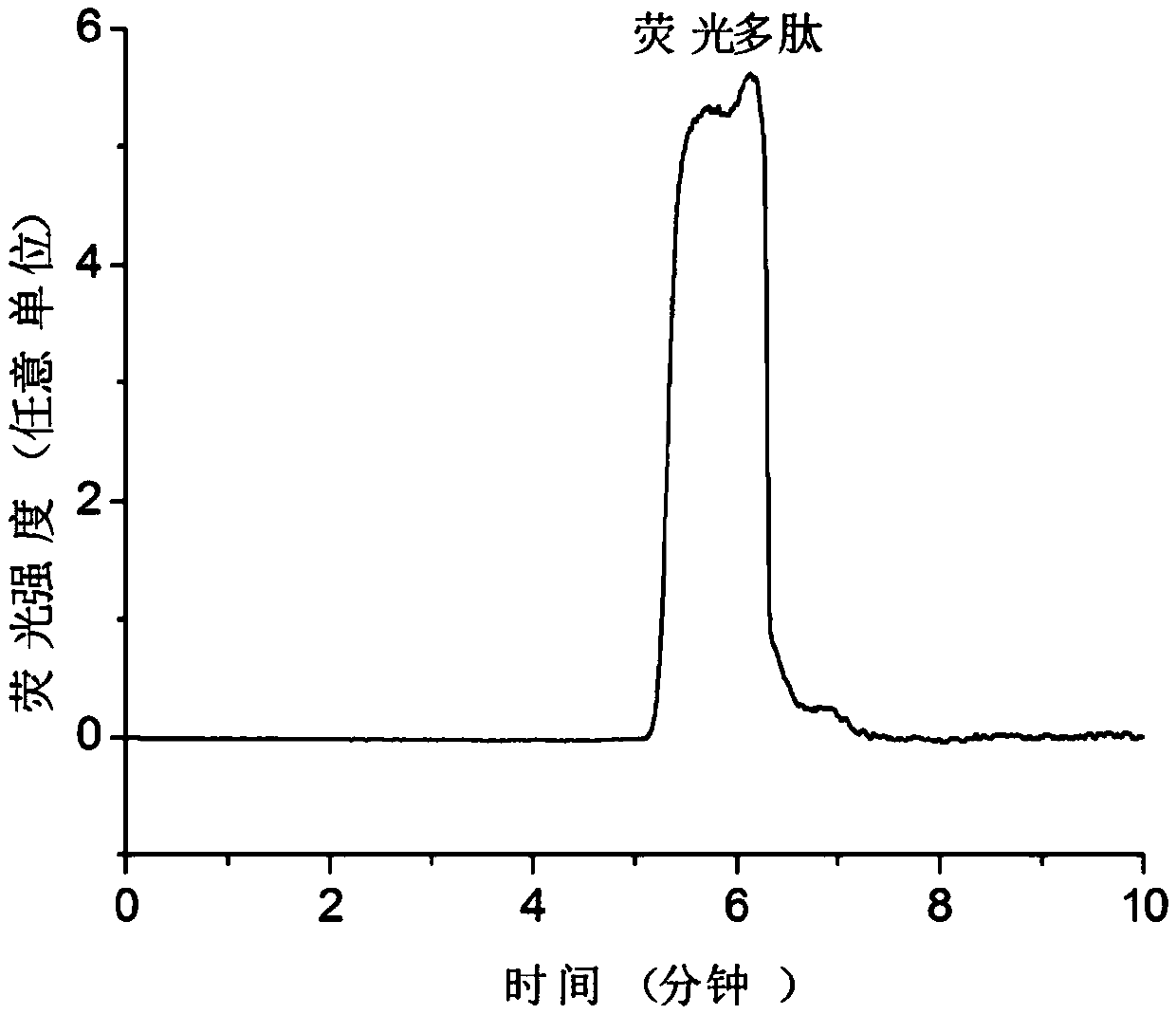
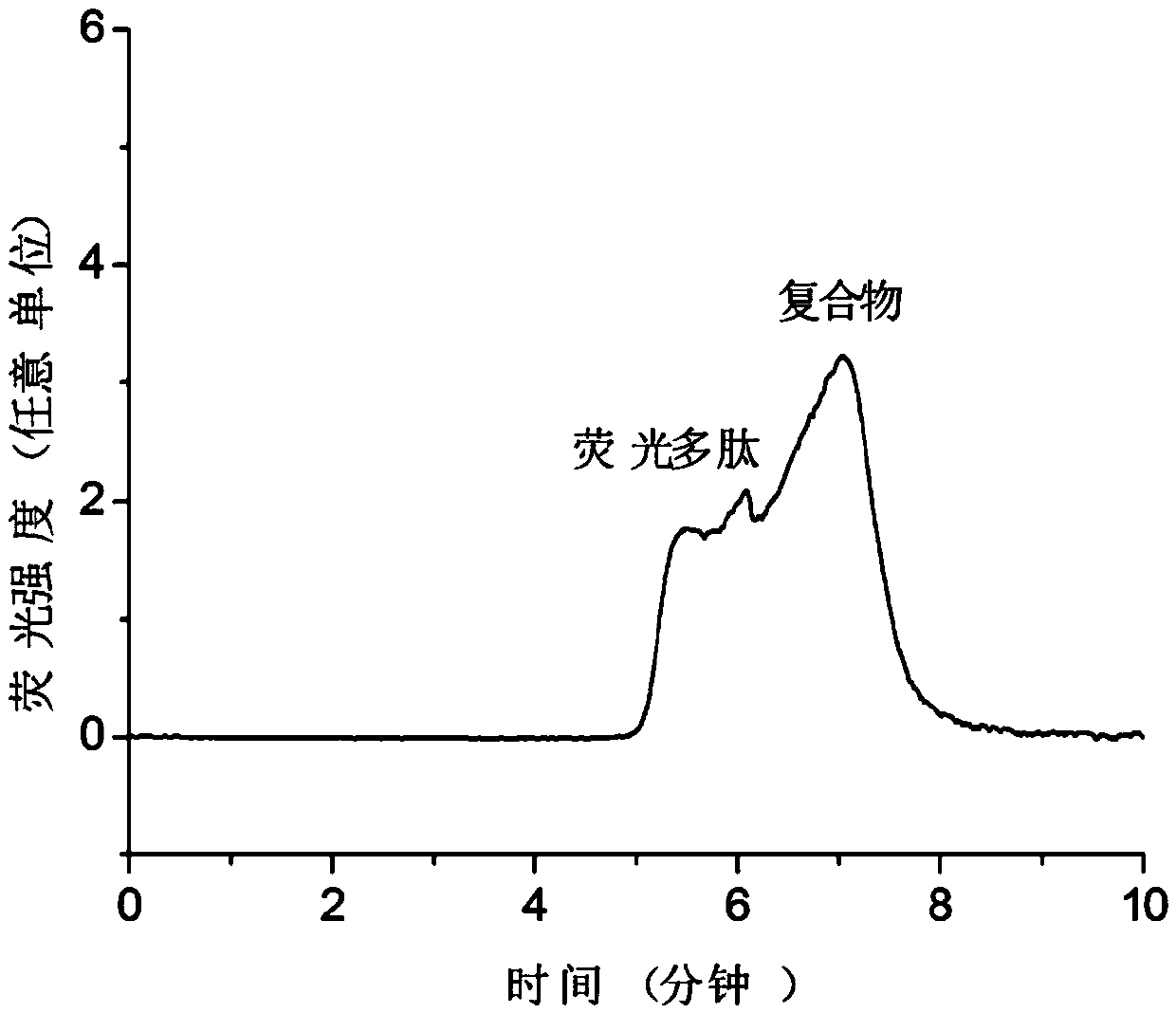
[0047] The polypeptide QEDIIRNIARHLAQVGDSMDRSIPPG (the sequence of the peptide is shown in SEQ ID NO: 1 in the sequence listing) is the BH3 region of the BID protein, which is synthesized by Jill Biochemical (Shanghai) Co., Ltd., and its N-terminus is subjected to 5-carboxyfluorescein (5 -FAM) labeling, in order to facilitate fluorescence detection and improve the sensitivity of detection, the peptide structure of the obtained substrate peptide is shown in formula E:
[0048]
[0049] The BCL-XL protein used in the examples is obtained after fusion expression of His tag and enzyme digestion and purification. ABT-737 was purchased from Selleckchem Company in the United States, with the structure shown in formula F, product number: S1002; norzeramaldehyde was purchased from Shanghai Chunyou Biotechnology Co., Ltd., product number: P0820; tripterygium was purchased from Shanghai A Latin Biochemical Technology Co., Ltd., product number: 1125828.
[0050]
[0051] Prepare 50...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com