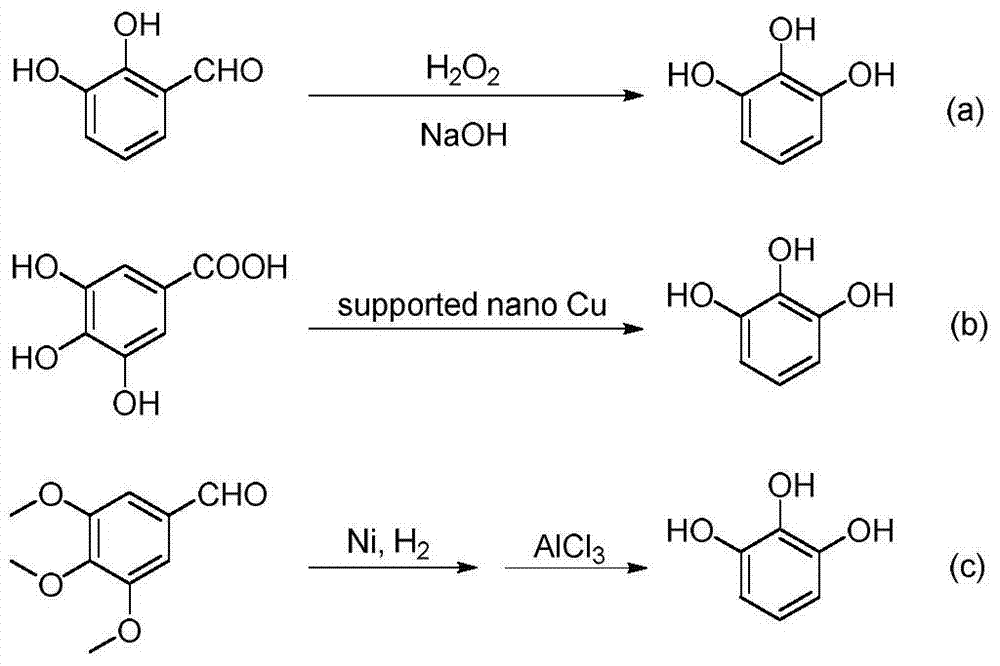
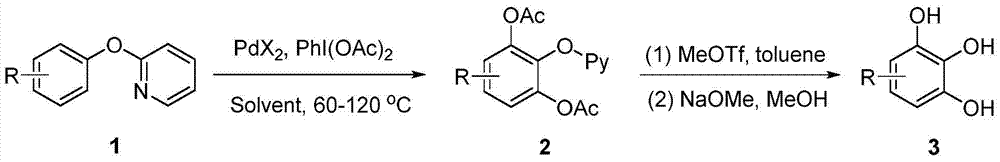
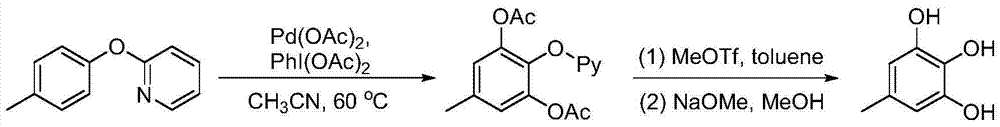
A kind of synthetic method of substituted pyrogallol
A synthesis method and pyrogallol technology are applied in the field of synthesis of substituted pyrogallol compounds, can solve the problems of short reaction steps, harsh raw material sources and the like, and achieve the effects of mild reaction conditions, easy products and high yields
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example 1
[0013] Example 1: Synthesis of 5-methylpyrogallol
[0014]
[0015] The mass ratio of the feed material 2-(4-methylphenoxy)pyridine: palladium acetate: iodobenzene diacetate is: 1:0.05:3, the organic solvent is acetonitrile, and the solvent consumption is 0.1mol / L (2-(4- methylphenoxy)pyridine molar concentration).
[0016] In a three-necked flask equipped with a thermometer, a reflux condenser and mechanical stirring, 1.85 g (0.01 mol) of 2-(4-methylphenoxy) pyridine, 0.11 g (0.5 mmol) of palladium acetate, and diacetic acid were added successively at room temperature. Iodobenzene 9.66g (0.03mol) and acetonitrile 100mL. After the addition, the temperature was raised to 60° C., and the reaction was maintained for 12 hours. After the reaction, the solvent was evaporated under reduced pressure, the residue was poured into ice water, extracted with ethyl acetate, washed with deionized water, and distilled under reduced pressure to obtain a crude product, which was recrystall...
example 2
[0019] Example 2: Synthesis of 5-chloropyrogallol
[0020]
[0021] The mass ratio of the feed material is 2-(4-chlorophenoxy)pyridine: palladium chloride: iodobenzene diacetate: 1:0.05:3, the organic solvent is N,N-dimethylacetamide, and the solvent consumption is 1mol / L (2-(4-chlorophenoxy)pyridine molar concentration).
[0022] In a three-necked flask equipped with a thermometer, a reflux condenser and mechanical stirring, 1.85 g (0.01 mol) of 2-(4-methylphenoxy) pyridine, 0.09 g (0.5 mmol) of palladium chloride, and di 9.66g (0.03mol) of iodobenzene acetate and 10mL of N,N-dimethylacetamide. After the addition was completed, the temperature was raised to 120° C., and the reaction was maintained for 3 hours. After the reaction, the solvent was evaporated under reduced pressure, the residue was poured into ice water, extracted with ethyl acetate, washed with deionized water, and distilled under reduced pressure to obtain a crude product, which was recrystallized from t...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com