Method of monitoring cellular trafficking of peptides
A cell and host cell technology, applied in the detection of programmed cell death, biochemical equipment and methods, biological testing, etc., can solve the problems of inability to select peptides, and cannot provide verification of cell internalization or delivery, etc., to reduce hydrophobicity Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0338] Generation of candidate peptide moieties
[0339] This example demonstrates the use of nucleic acids encoding candidate peptides to generate candidate peptide moieties, eg, peptide libraries such as bacteriophage display libraries or other peptide display scaffolds.
[0340] High diversity mixtures of nucleic acids encoding candidate peptides are generated from the coding and non-coding regions of bacterial genomes and eukaryotes with compact genomes, as essentially described in U.S. Patent No. 7,270,969, and as described below, the selected source genome can vary , and the vector used for expression of the peptide encoded by the nucleic acid described in the Examples below may vary. The contents of US Patent No. 7,270,969 are incorporated herein by reference in their entirety.
[0341] Briefly, nucleic acids were isolated from the following bacterial and archaeal species:
[0342]
[0343]
[0344] Nucleic acid fragments were generated separately from these gen...
Embodiment 2
[0353] Production of non-biotinylated fractions using the expression vector pNp3
[0354] This example demonstrates the production of non-biotinylated components using the expression vector pNp3 or derivatives thereof to obtain filamentous bacteriophage displaying non-biotinylated components.
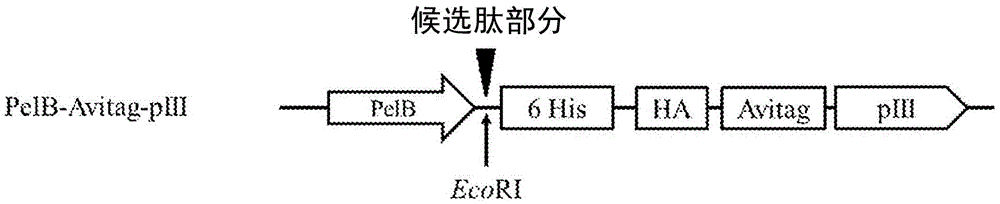
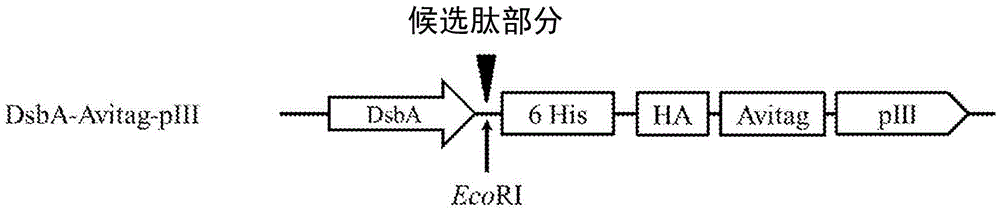
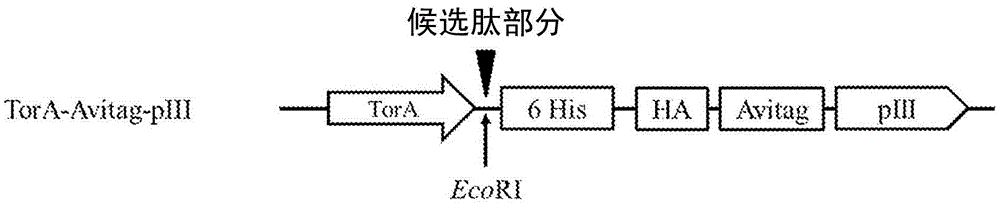
[0355] The designated vector construct, pNp3, is an M13 vector comprising an encoded fusion protein containing a hexahistidine (6His) tag, a hemagglutinin (HA) tag, a biotin ligase substrate domain, and the M13pIII coat protein. Vector pNp3 was modified to express a fusion protein comprising a candidate peptide moiety fused in-frame with a 15-amino acid biotin ligase substrate domain having the amino acid sequence shown in SEQ ID NO: 4, as shown in Figure 1a, 1b and 1c are shown. Fusion proteins generated using pNp3 were subsequently displayed on scaffolds containing filamentous bacteriophage M13.
[0356] Figure 1a shows the pIII fusion protein encoded by the pNp3 derivative vector P...
Embodiment 3
[0376] Production of non-biotinylated fractions using the expression vector pNp8
[0377] This example demonstrates the production of non-biotinylated components using the expression vector pNp8 or derivatives thereof to obtain filamentous bacteriophage displaying non-biotinylated components.
[0378] The designated vector construct, pNp8, is an M13 vector comprising a fusion protein encoding a hexahistidine (10His) tag, a hemagglutinin (HA) tag, a biotin ligase substrate domain, and the M13pVIII coat protein. Vector pNp8 was modified to express a fusion protein comprising a candidate peptide moiety fused in-frame to a 15-amino acid biotin ligase substrate domain having the amino acid sequence shown in SEQ ID NO: 4, as shown in Figures 4a and 4b. Fusion proteins generated using pNp8 were subsequently displayed on scaffolds containing filamentous bacteriophage M13.
[0379] Figure 4a shows the pVIII fusion protein encoded by the pNp8 derivative vector PelB-Avitag-pVIII, which ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com