Bifidobacterium longum protein, and preparation method and medical application thereof
A bifidobacterium longum and protein technology, applied in the field of microorganisms, can solve problems such as singleness and lack of ingredients, and achieve the effects of expanding the scope of use, improving sensitivity, and improving microbial drug sensitivity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0042] Embodiment 1 (recombinant escherichia coli expression strain that prepares Bifidobacterium longum protein)
[0043] 1.1 Experimental materials
[0044] 1.1.1 Strains and vectors
[0045] Bifidobacterium longum, Escherichia coli DH5α, and vector pGEX-4T-1 were all purchased from the market.
[0046] 1.1.2 Commonly used reagents
[0047] (1) TE buffer (pH8.0): 10mmol / LTris-HCl (pH8.0), 1mmol / LEDTANa 2 (pH8.0). After configuration, autoclave and store at room temperature.
[0048] (2) 10mg / mL lysozyme: 100mg lysozyme dissolved in 10mLddH 2 O, stored at –20°C for later use.
[0049] (3) 10% SDS: 5gSDS plus ddH 2 O was dissolved and the volume was adjusted to 50 mL.
[0050] (4) 3MKAc: 14.72gKAc dissolved in ddH 2 O and make up to 50mL.
[0051] (5) 3MNaAc: 12.35gNaAc dissolved in ddH 2 O and make up to 50mL.
[0052] (6) 0.1MCaCl 2 : 11.1gCaCl 2 Dissolve in 1000mLddH 2 In O, autoclave at 121°C for 15 minutes, and store at 4°C.
[0053] (7) 50×TAE electrophor...
Embodiment 2
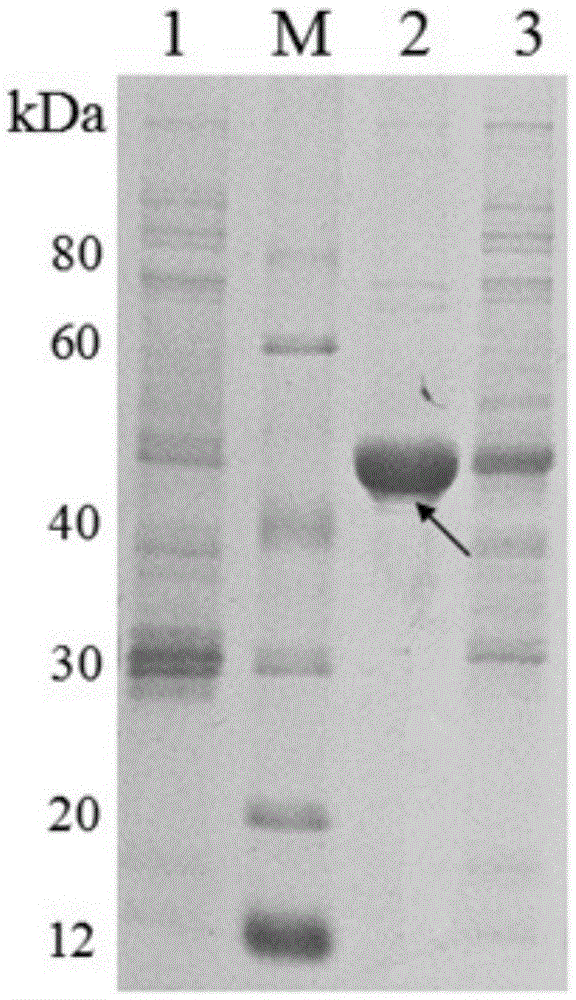
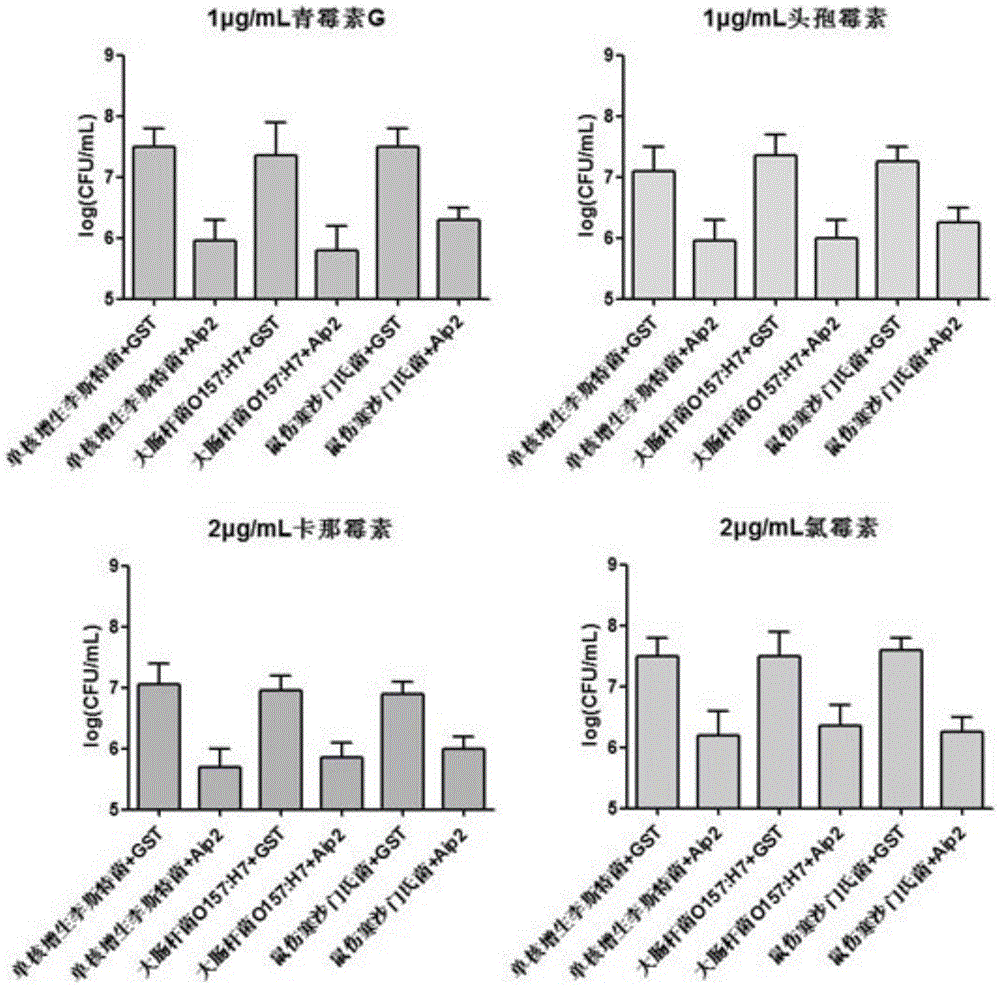
[0160] Example 2 (Induced expression and purification of Bifidobacterium longum adhesion protein)
[0161] Step 1: Induced expression of Bifidobacterium longum adhesion protein
[0162] The Bifidobacterium longum adhesion protein expression strain E.coliDH5αpGEX-4T-AIP2 prepared in Example 1 was induced and expressed with IPTG, and the specific steps were as follows:
[0163] (1) Pick a single colony of the strain that has been streaked on the plate, inoculate it into 3ml of LB liquid medium, add Amp to the test tube to a final concentration of 100μg / ml, and culture it at 37°C with a constant temperature shaker at 180r / m for 8h.
[0164] (2) Transfer to 20ml low-salt LB liquid medium according to 1% inoculum size, add Amp to a final concentration of 100μg / ml, and culture at 37°C with a constant temperature shaker at 180r / m.
[0165] (3) When the cell density OD600nm = 0.6-0.9, add the inducer IPTG to a final concentration of 1 mM, incubate at 37° C. with a constant temperatur...
Embodiment 3
[0186] Embodiment 3 (a kind of aminoacid sequence is the protein preparation method of SEQIDNO:1)
[0187] 1) Take recombinant Escherichia coli E.coli DH5αpGEX-4T-AIP2 and culture it. When the OD600nm of the culture medium is 0.9, add the inducer IPTG to a final concentration of 1.2mM, and then induce culture at 39°C for 5 hours under shaking conditions;
[0188] 2) collecting the bacteria, crushing, and collecting the supernatant;
[0189] 3) Take buffer A, equilibrate the glutathione agarose resin column with a flow rate of 3.5mL / min; take the supernatant from step 2), load the sample with a flow rate of 2mL / min; take buffer B, The flow rate of min is eluted, and the solution at the elution peak is collected as the eluent; the eluent is collected by ultrafiltration and concentrated, then passed through a desalting column, and then eluted with pure water at a flow rate of 3.5mL / min, and the eluent at the elution peak is collected. The solution is the second eluent, and then ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com