Recombinant human insulin genetically engineered bacterium high expression strain selection culture medium and preparation method
A technology of recombinant human insulin and genetically engineered bacteria, which is applied in the direction of insulin, microbial-based methods, biochemical equipment and methods, etc., can solve the problems of single nutrient composition in the culture medium, increased pollution probability, complicated screening process, etc., to reduce pollution , screening stability, and improved screening method performance
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
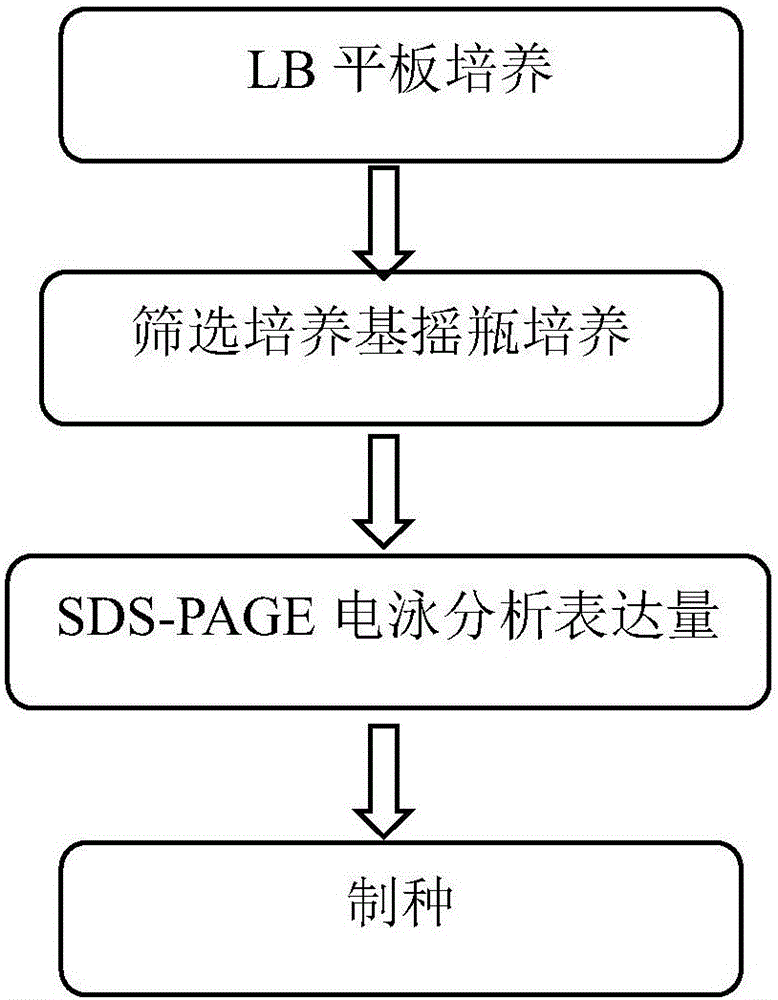
[0033] according to figure 1 Precisely prepare 1 liter of recombinant human insulin genetically engineered bacteria high-expression strain screening medium, each component and its quality are: peptone 1.5g, yeast powder 3g, potassium dihydrogen phosphate 1.5g, disodium hydrogen phosphate 4.5g , sodium chloride 0.3g, ammonium chloride 0.1g, adjust the pH to 6.80-7.20, and the balance is purified water.
[0034] Weigh the raw materials of each component according to the stated ratio, dissolve them in purified water to 90% of the final volume, adjust the pH to 6.80-7.20, after constant volume, sterilize at 121.0°C for 30 minutes, add sterile kanamycin to a final concentration of 40mg / L.
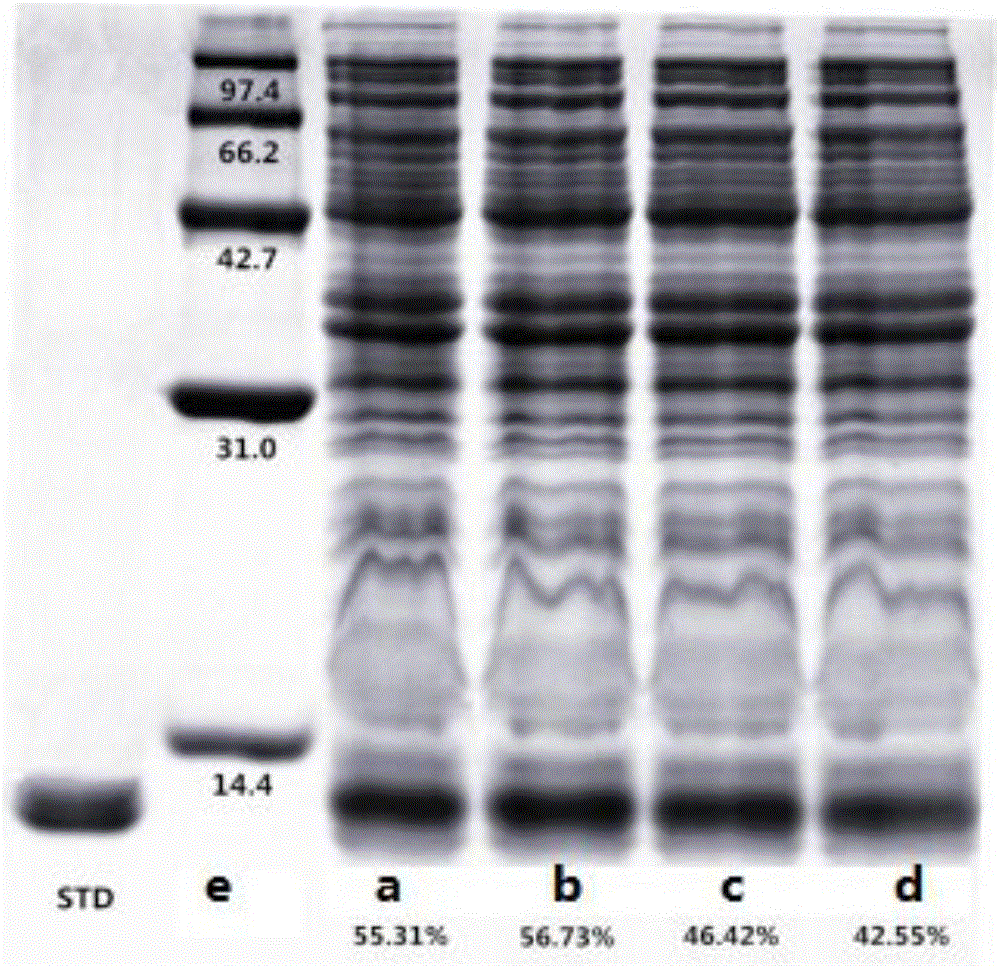
[0035] Prepare the screening medium for the high-expression strain of recombinant human insulin genetically engineered bacteria, using existing recombinant human insulin genetically engineered bacteria (host bacteria is E.colik12W3110, expression plasmid is PL-82, containing kanamycin (Kana) a...
Embodiment 2
[0037] according to figure 1 According to the process, 1 liter of recombinant human insulin genetically engineered bacteria high-expression strain screening medium was precisely prepared. The components and their quality were: 3g of peptone, 6g of yeast powder, 3.5g of potassium dihydrogen phosphate, 6.5g of disodium hydrogen phosphate, Sodium chloride 0.6g, ammonium chloride 0.3g, adjust the pH to 6.80-7.20, and the balance is purified water.
[0038] Weigh the raw materials of each component according to the stated ratio, dissolve them in purified water to 90% of the final volume, adjust the pH to 6.80-7.20, after constant volume, sterilize at 121.0°C for 30 minutes, add sterile kanamycin to a final concentration of 30mg / L.
[0039] Prepare the screening medium for the high-expression strain of recombinant human insulin genetically engineered bacteria, using the existing recombinant human insulin genetically engineered bacteria (host bacteria is E.colik12W3110, expression ...
Embodiment 3
[0041] according to figure 1 According to the process, 1 liter of recombinant human insulin genetically engineered bacteria high-expression strain screening medium was precisely prepared. The components and their mass fractions were: peptone 2g, yeast powder 5g, potassium dihydrogen phosphate 2.5g, disodium hydrogen phosphate 5g, chlorine 0.5 g of sodium chloride and 0.2 g of ammonium chloride were used to adjust the pH to 7, and the balance was purified water.
[0042] Weigh the raw materials of each component according to the stated ratio, dissolve them in purified water to 90% of the final volume, adjust the pH to 6.80-7.20, after constant volume, sterilize at 121.0°C for 30 minutes, add sterile kanamycin to a final concentration of 40mg / L.
[0043] Prepare the screening medium for the high-expression strain of recombinant human insulin genetically engineered bacteria, using the existing recombinant human insulin genetically engineered bacteria (host bacteria is E.colik12...
PUM
 Login to view more
Login to view more Abstract
Description
Claims
Application Information
 Login to view more
Login to view more - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap