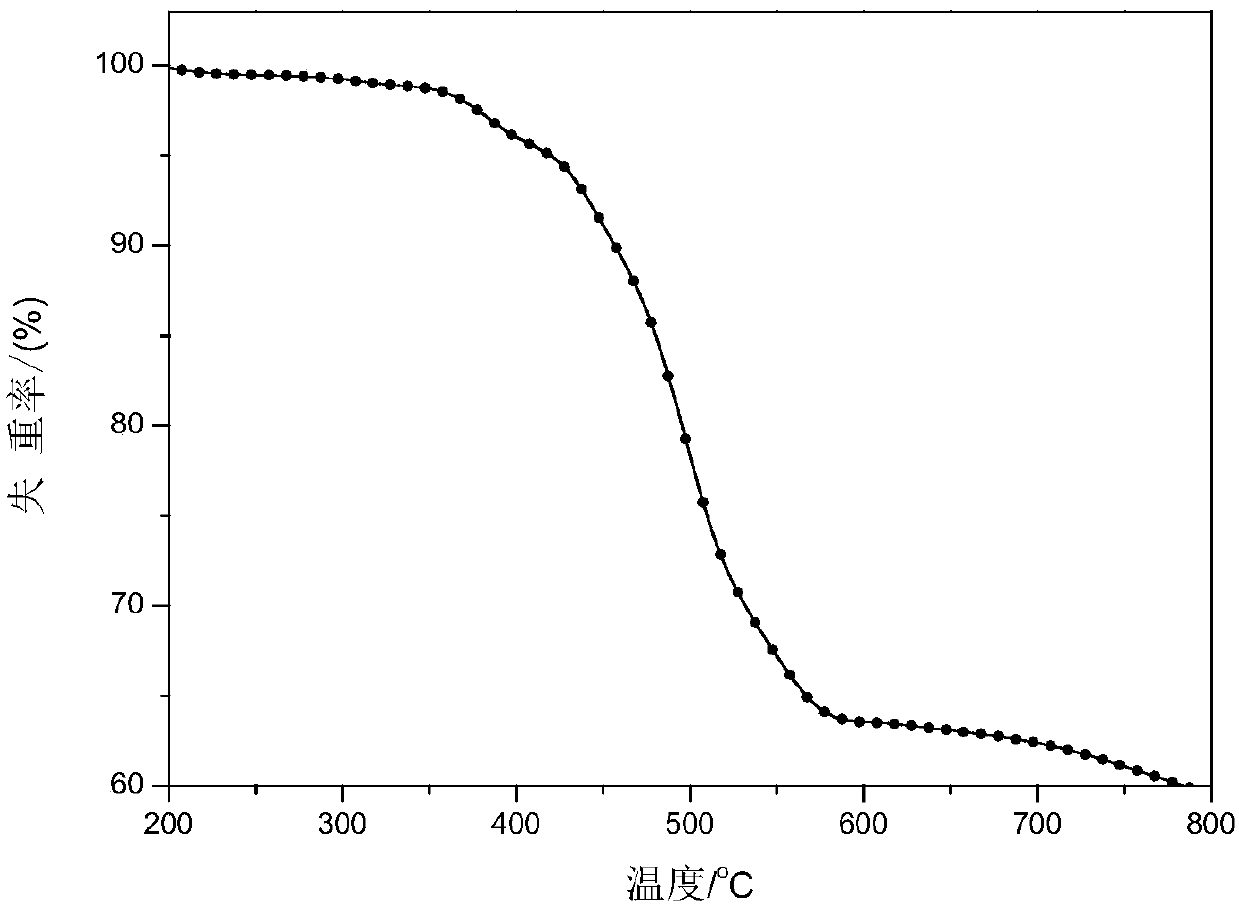
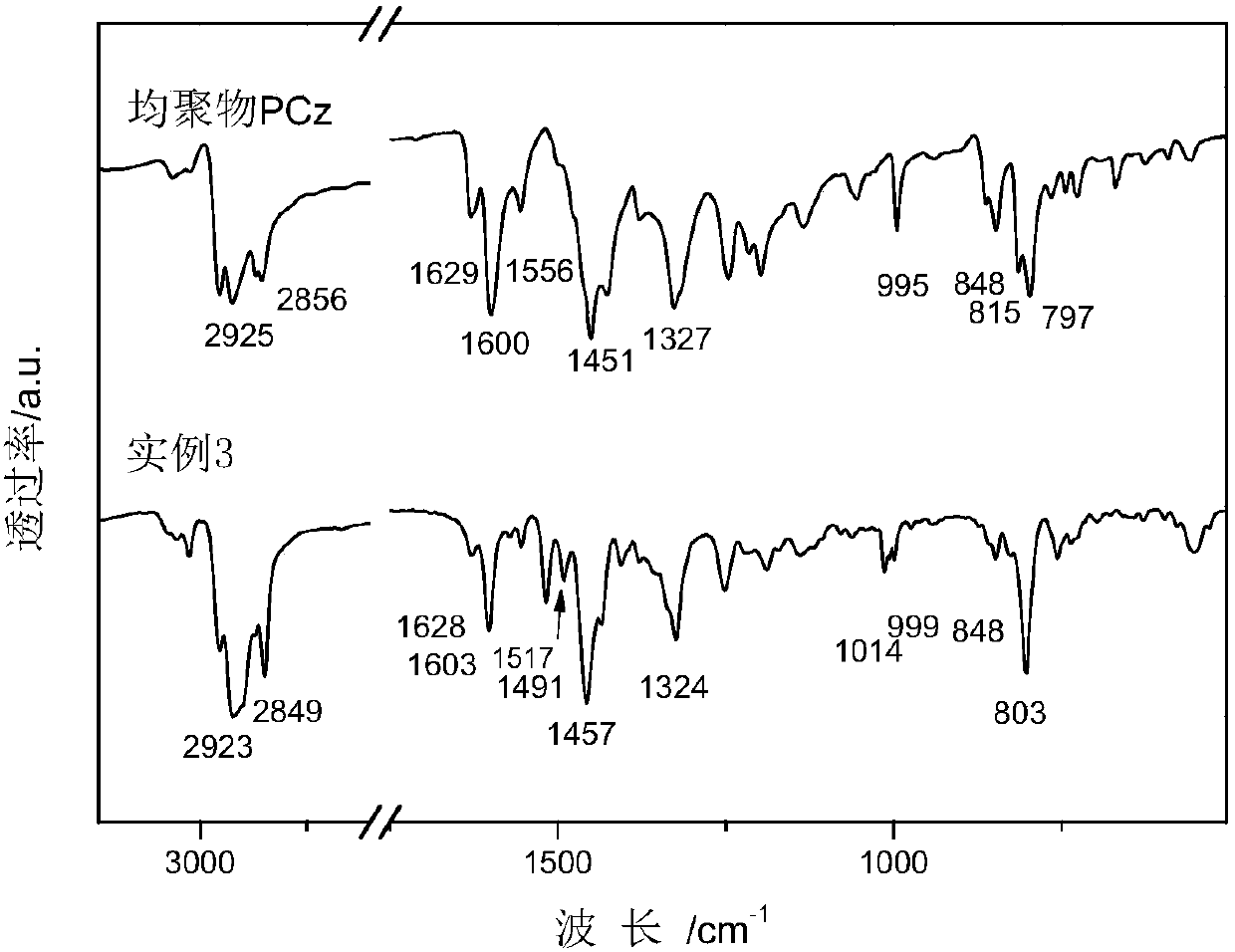
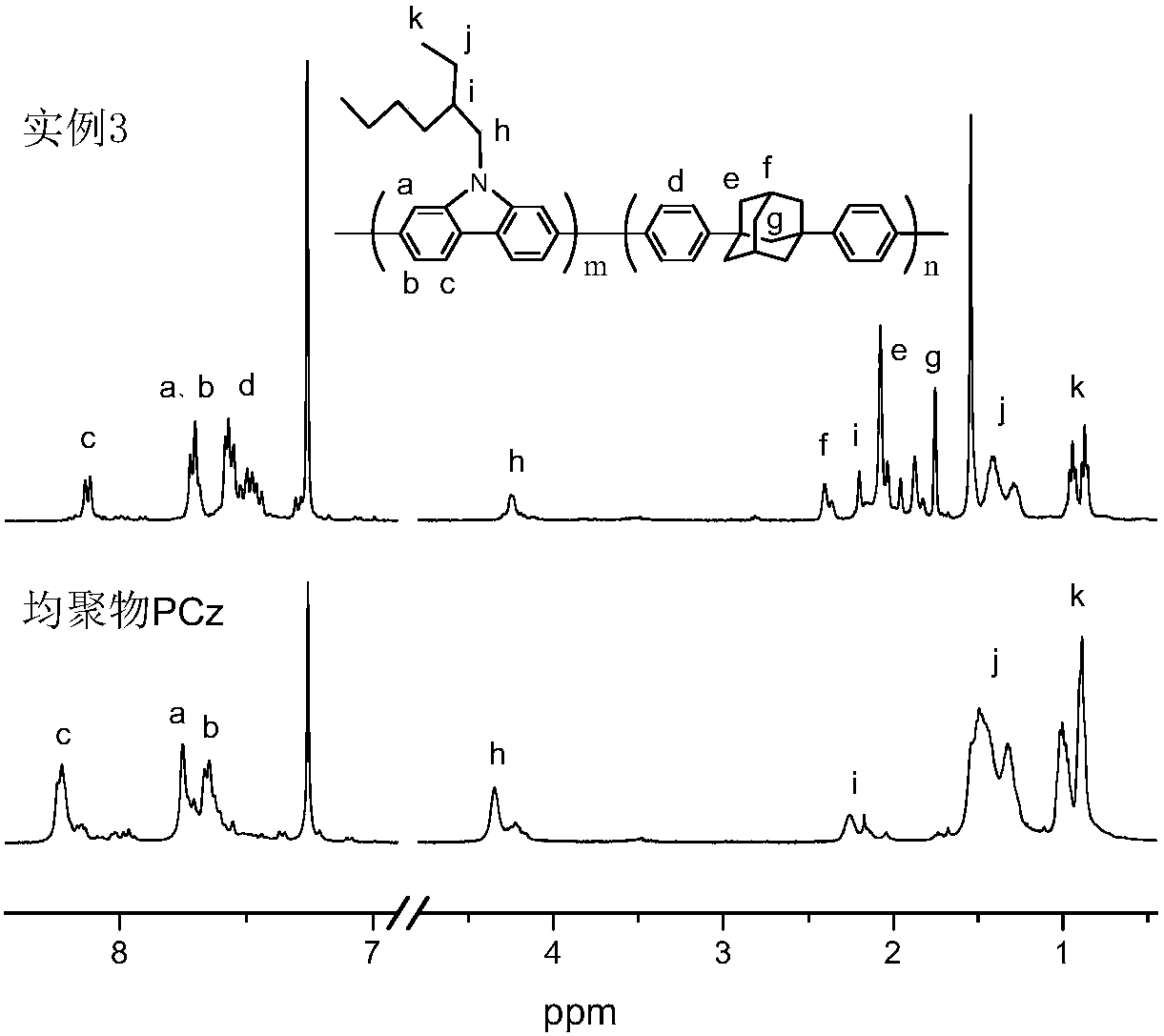
A kind of adamantane modified polycarbazole polymer luminescent material and preparation method thereof
A technology of azole polymer and light-emitting material, which is applied in the field of adamantane-modified polycarbazole polymer light-emitting material and its preparation, can solve the problems of limiting the application range of carbazole material, poor device stability, unstable light emission, etc. Possibility of fluorescence quenching, convenient film formation, and excellent solubility
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0055] (1) Preparation of 4,4'-dibromo-2-nitrobiphenyl
[0056] 4,4'-Dibromobiphenyl (2g, 6.41mmol) and glacial acetic acid (30ml) were added into the reaction flask, stirred by magnetic force, and the temperature of the oil bath was raised to 100°C. Through a constant-pressure dropping funnel, dropwise add a mixed solution of distilled water (0.75mL) and fuming nitric acid (9.25mL), continue stirring at 100°C for 0.5h, then pour into 500mL of ice water, stir, suction filter, and vacuum-dry the solid. The solid was recrystallized from ethanol to obtain 4,4'-dibromo-2-nitrobiphenyl as a light yellow solid with a yield of 68.2%;
[0057] (2) Preparation of 2,7-dibromocarbazole
[0058] Add 4,4'-dibromo-2-nitrobiphenyl (2g, 5.60mmol) and triphenylphosphine (4.40g, 16.80mmol) of step (1) into a 250mL three-necked round-bottomed flask, and add the reaction solvent o-dichlorobenzene 125mL, stirred vigorously, and raised the temperature to 170°C to reflux, reacted for 9h, cooled, a...
Embodiment 2
[0072] (1) Preparation of 4,4'-dibromo-2-nitrobiphenyl
[0073] 4,4'-Dibromobiphenyl (2g, 6.41mmol) and glacial acetic acid (30ml) were added into the reaction flask, stirred by magnetic force, and the temperature of the oil bath was raised to 100°C. A mixed solution of distilled water (0.62 mL) and fuming nitric acid (9.25 mL) was added dropwise through a constant pressure dropping funnel. After continuing stirring at 100° C. for 0.5 h, pour into 500 mL of ice water, stir, filter with suction, and dry the solid in vacuo. The crude product was recrystallized from ethanol to obtain 4,4'-dibromo-2-nitrobiphenyl as a light yellow solid with a yield of 60.2%.
[0074] (2) Preparation of 2,7-dibromocarbazole
[0075] 4,4'-Dibromo-2-nitrobiphenyl (2g, 5.60mmol) and triphenylphosphine (4.00g, 15.30mmol) were added into a 250mL three-neck round bottom flask. 125 mL of the reaction solvent o-dichlorobenzene was added, stirred vigorously, and heated to 170° C. to reflux. After react...
Embodiment 3
[0088] (1) Preparation of 4,4'-dibromo-2-nitrobiphenyl
[0089] 4,4'-Dibromobiphenyl (2g, 6.41mmol) and glacial acetic acid (30ml) were added into the reaction flask, stirred by magnetic force, and the temperature of the oil bath was raised to 100°C. A mixed solution of distilled water (0.93 mL) and fuming nitric acid (9.25 mL) was added dropwise through a constant pressure dropping funnel. After continuing stirring at 100° C. for 0.5 h, pour into 500 mL of ice water, stir, filter with suction, and dry the solid in vacuo. The crude product was recrystallized from ethanol to obtain 4,4'-dibromo-2-nitrobiphenyl as a light yellow solid with a yield of 62.8%.
[0090] (2) Preparation of 2,7-dibromocarbazole
[0091] 4,4'-Dibromo-2-nitrobiphenyl (2g, 5.60mmol) and triphenylphosphine (6.00g, 22.91mmol) were added into a 250mL three-neck round bottom flask. 125 mL of the reaction solvent o-dichlorobenzene was added, stirred vigorously, and heated to 170° C. to reflux. After react...
PUM
| Property | Measurement | Unit |
|---|---|---|
| thermal decomposition temperature | aaaaa | aaaaa |
| thermal decomposition temperature | aaaaa | aaaaa |
| thermal decomposition temperature | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com