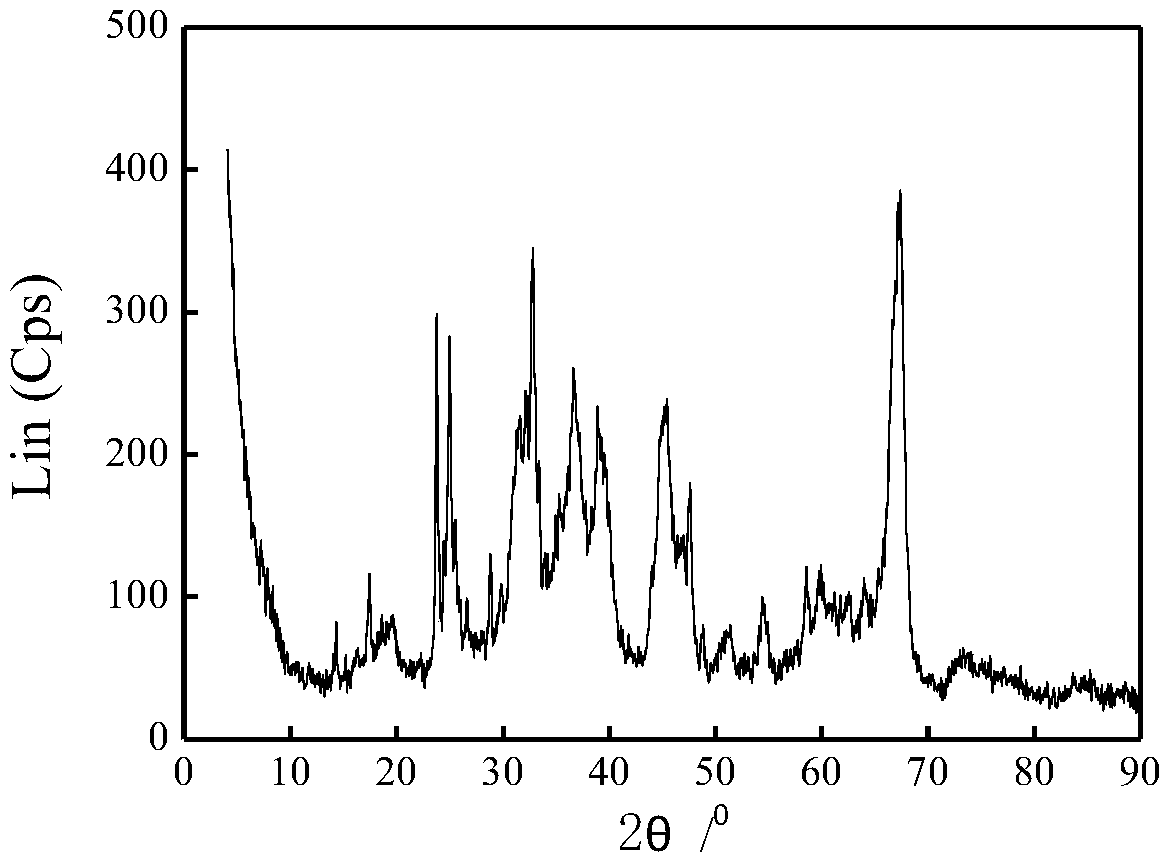
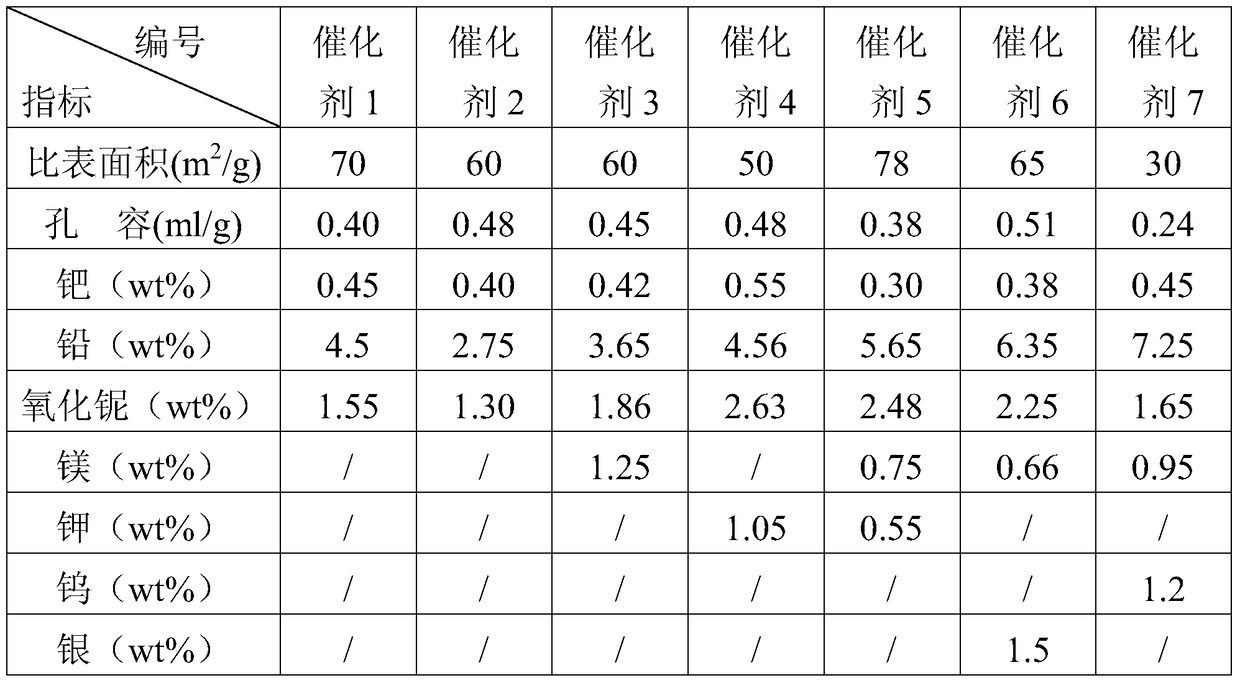
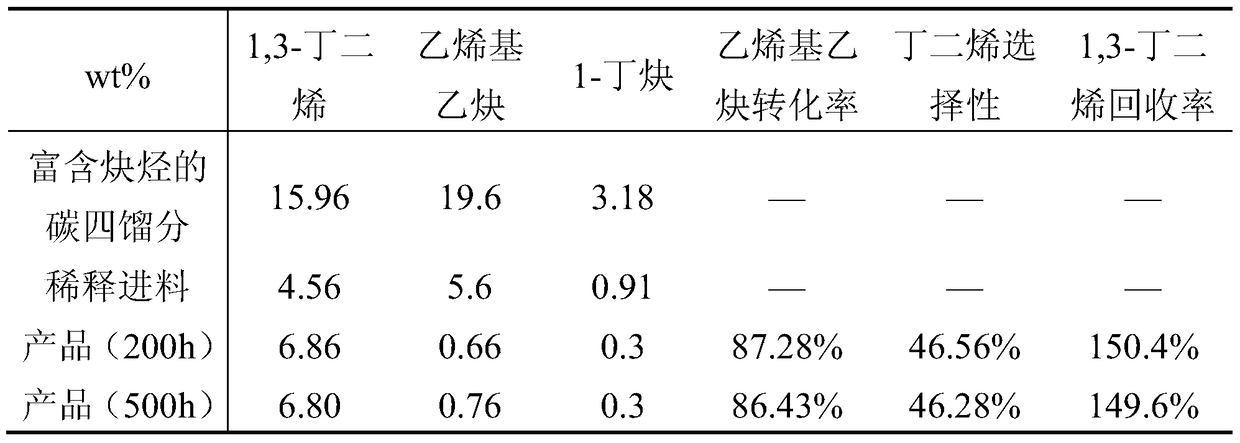
A kind of method of alkyne selective hydrogenation
A selective and alkyne-based technology, applied in chemical instruments and methods, purification/separation of hydrocarbons, hydrogenation to hydrocarbons, etc., can solve the problems of high product circulation ratio, low liquid space velocity, etc., to improve thermal stability , Improve the acid properties, increase the output effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0062] The C4 fraction rich in alkynes is diluted with the C4 raffinate, and the weight ratio of the C4 fraction rich in alkynes to the C4 raffinate is 1:2.5. The adiabatic reactor adopts a single-stage adiabatic bubbling bed, and uses catalyst 1, and the catalyst is reduced at 120°C for 6 hours under a hydrogen atmosphere. Reaction temperature 50℃, reaction pressure 1.2MPa, liquid space velocity 8.5h -1 , the molar ratio of hydrogen to alkyne is 1.5, Table 2 is the composition of materials before and after the reaction.
[0063] Table 2 Composition of materials before and after reaction
[0064]
Embodiment 2
[0066] The C4 fraction rich in alkynes is diluted with the C4 raffinate, and the weight ratio of the C4 fraction rich in alkynes to the C4 raffinate is 1:3. The adiabatic reactor adopts a single-stage adiabatic bubbling bed, adopts catalyst 2, and the catalyst is reduced at 120°C for 6 hours under a hydrogen atmosphere. The reaction temperature is 45°C, the reaction pressure is 1.5MPa, and the liquid space velocity is 9.0h -1 , the molar ratio of hydrogen to alkyne is 1.5, Table 3 is the composition of materials before and after the reaction.
[0067] Table 3 Composition of materials before and after reaction
[0068]
Embodiment 3
[0070] Dilute the alkyne-rich C4 fraction with the raffinate C4, the weight ratio of the alkyne-rich C4 fraction to the raffinate C4 is 1:3, and the adiabatic reactor adopts a single-stage adiabatic bubbling bed, Catalyst 3 was used, and the catalyst was reduced at 120° C. for 6 h under hydrogen atmosphere. The reaction temperature is 45°C, the reaction pressure is 2.0MPa, and the liquid space velocity is 10.0h -1 , the molar ratio of hydrogen to alkyne is 2.0, Table 4 is the composition of materials before and after the reaction.
[0071] Table 4 Composition of materials before and after reaction
[0072]
PUM
| Property | Measurement | Unit |
|---|---|---|
| specific surface area | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com