A kind of Mirabegron sustained-release tablet and preparation method thereof
A technology of mirabegron and sustained-release tablets, which is applied in the direction of pharmaceutical formulas, medical preparations containing no active ingredients, and medical preparations containing active ingredients. Incomplete release and other problems, to achieve convenient clinical use, good clinical application prospects, and increase the effect of compliance
- Summary
- Abstract
- Description
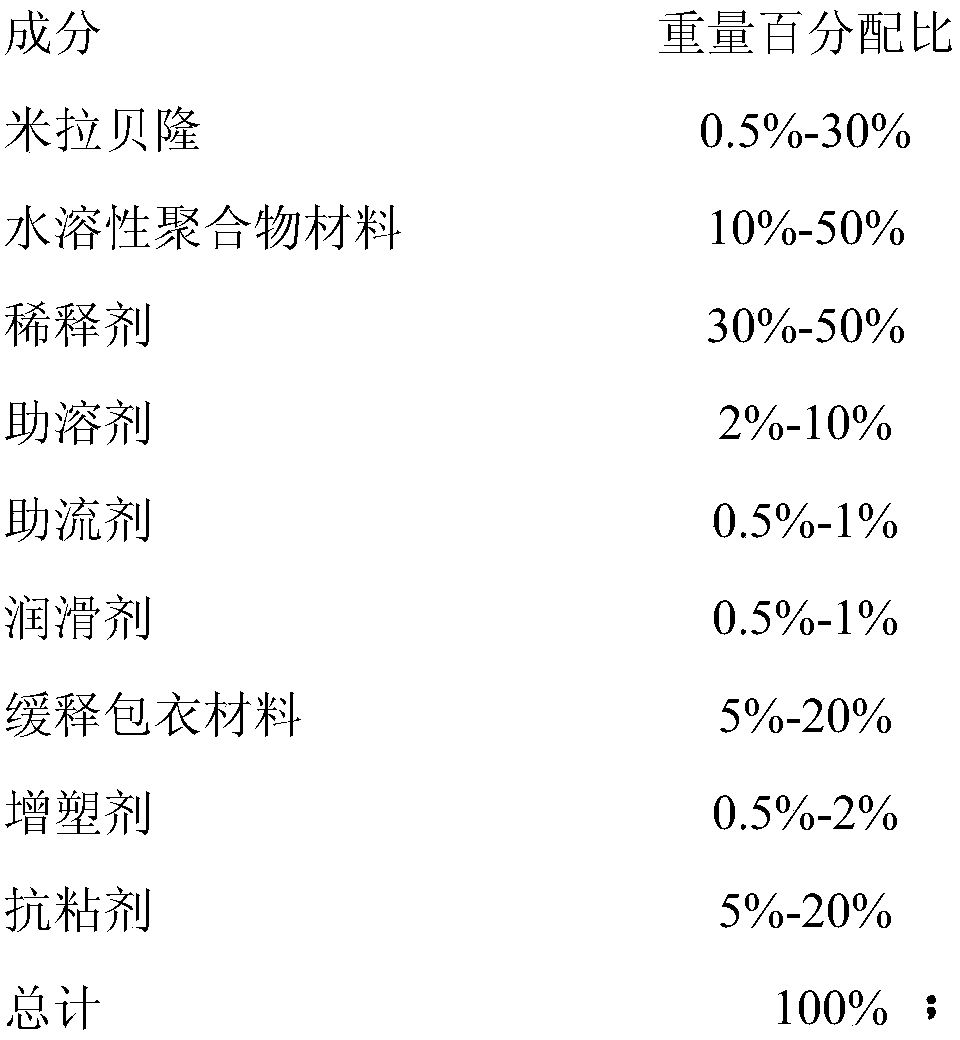
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
total
686
[0039] Preparation steps: take 180g mirabegron, 70g hydroxypropyl methylcellulose E6 and 30g sodium lauryl sulfate and carry out micronization with an airflow mill, the airflow pressure is 2.0Mpa, the feed rate is 500g / h, pulverize After measuring the particle size, the D90 is 8.9um; put the above micronized material into the hot melt granulator KJL-100, heat to 150°C, stir evenly, add 28g of 80°C hot water to granulate, and make wet granules. Cool to 28°C while stirring, and determine that the weight loss on drying is 3.4%; add microcrystalline cellulose, silicon dioxide and magnesium stearate, stir for 5 minutes, and mix well to obtain the final mixture before tableting; use Fette to tablet Machine 102i compresses the mixture into tablets to obtain 6,000 Mirabegron tablets; coats the aqueous dispersion prepared from polyacrylic acid resin NE30D, triethyl citrate and talcum powder to obtain sustained-release tablets. The tablet after coating was aged ...
Embodiment 2
total
900
[0048] Preparation steps: take 240g mirabegron, 200g hydroxypropyl cellulose and 30g vitamin E TPGS and use a jet mill to micronize, the air pressure is 2.0Mpa, the feed rate is 700g / h, and the particle size is measured after the pulverization, D90 8.0um; put the above micronized material into a hot-melt granulator, heat to 180°C, stir evenly, add 55g of 80°C hot water to granulate, and make wet granules, cool to 25°C while stirring, measure the dryness The weight loss is 2.4%; add lactose, silicon dioxide and magnesium stearate, stir for 10 minutes, and mix well to obtain the final mixture before tableting; use the Fette tablet machine to compress the mixture to obtain Mirabegron tablets 6000 tablets; sustained-release tablets are prepared by coating an aqueous dispersion prepared from ethyl cellulose, triethyl citrate and talcum powder. The coated tablet was aged at 40°C for 48 hours to obtain the final Mirabegron sustained-release tablet.
[0049] ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com