Azithromycin injection for veterinary use
A technology of azithromycin and injection, applied in the field of medicinal chemistry, can solve the problems of strong irritation, easy infusion reaction, poor compliance, etc., and achieve the effect of less irritation, favorable application and long acting time
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
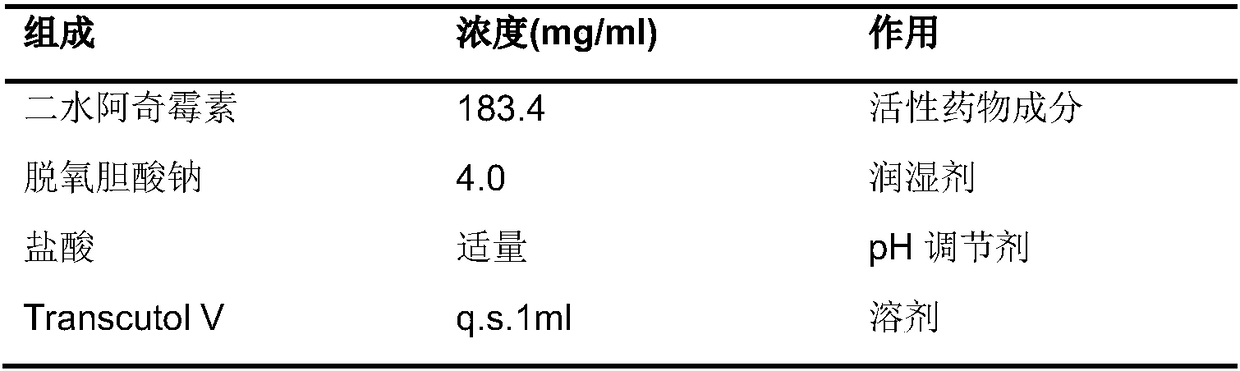
[0019] The preparation method of the aforementioned solution-type injection may include the following steps: dissolving the prescribed amount of active pharmaceutical ingredients in diethylene glycol monoethyl ether, stirring and dissolving; subpackaging, plugging, capping, and packaging.
[0020] On the other hand, the veterinary azithromycin injection provided by the present invention can be a lyophilized injection.
[0021] The freeze-dried injection includes active pharmaceutical ingredients and sodium deoxycholate, wherein the active pharmaceutical ingredients are as defined above.
[0022] The particle size of the active pharmaceutical ingredient of the freeze-dried injection has the following characteristics: its D50 is 1 μm-10 μm, and its D90 is 3 μm-15 μm. In some embodiments, the particle size of the active pharmaceutical ingredient of the freeze-dried powder has the following characteristics: its D50 is 4 μm-6 μm, and its D90 is 9 μm-12 μm. Too large particle size ...
specific Embodiment approach
[0037] In order to enable those skilled in the art to better understand the technical solutions of the present invention, some non-limiting examples are further disclosed below to further describe the present invention in detail.
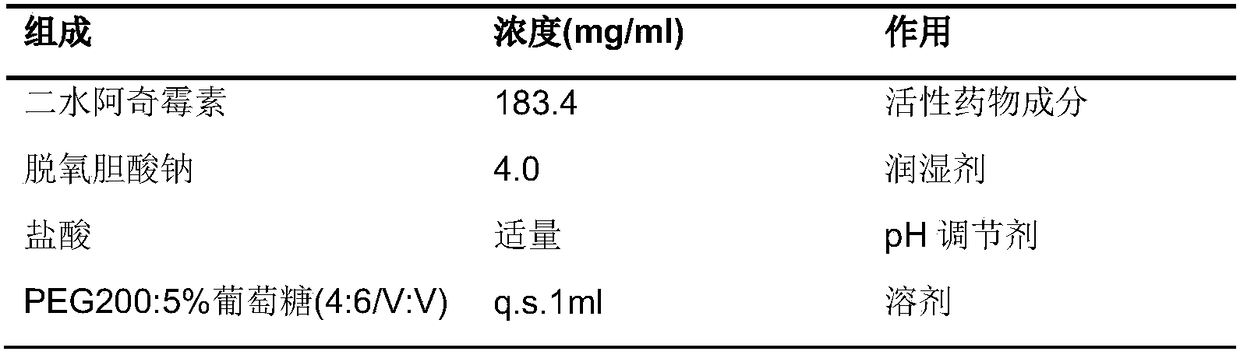
[0038] The reagents used in the present invention can be purchased from the market or can be prepared by the methods described in the present invention. The source of Diethylene Glycol Monoethyl Ether (Transcutol V) is Jaffa Lion, and "V" is the model number, representing veterinary use.
[0039] In the present invention, kg: kilogram; g: gram; mg: milligram; ng: nanogram; mL: milliliter; min: minute; h: hour; q.s.: constant volume; PEG: polyethylene glycol; AUC: drug-time curve Cmax: peak concentration; Tmax: peak time; MIC: minimum inhibitory concentration; SD: standard deviation; CV: coefficient of variation; The area under the drug-time curve is calculated by the corresponding software.
[0040] Freeze-drying method and conditions of the prese...
Embodiment 1
[0042] Preparation of Comparative Example 1 Azithromycin Injection Suspension
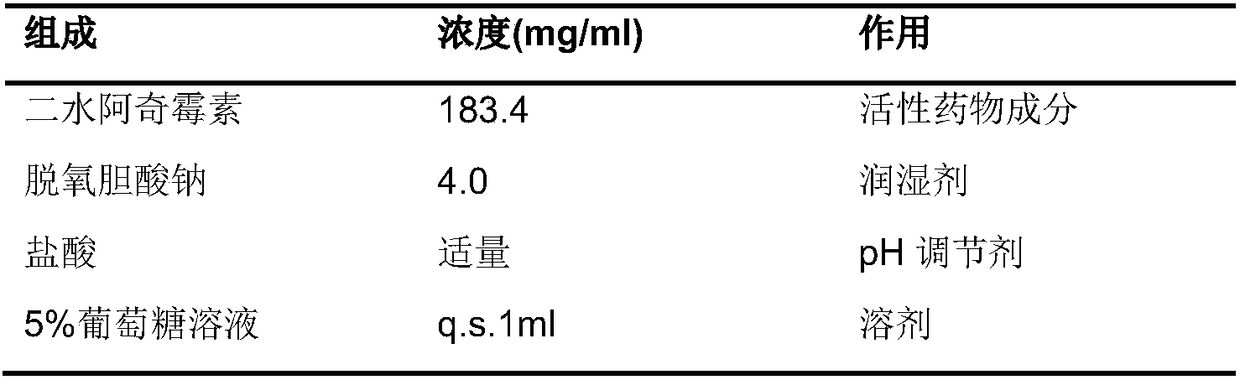
[0043] To prepare azithromycin injection suspension, the prescription is as follows:
[0044]
[0045] Preparation method: Add azithromycin dihydrate and sodium deoxycholate to water for injection, grind for 15 minutes with a ball mill, adjust the pH value to 8.55 with hydrochloric acid, absorb the suspension for injection, and freeze-dry it with a vacuum freeze dryer to obtain a freeze-dried powder. The lyophilized powder is reconstituted with a certain amount of 5% glucose to obtain an injection suspension.
[0046] The resulting suspension was detected, and the particle size of the active ingredient in the suspension was measured as D50 of 5.77 μm and D90 of 11.9 μm; the suspension had a uniform appearance, good fluidity, slow settling, and good redispersibility. Non-stick to the wall, good needle penetration, suitable for injection. After the lyophilized powder is reconstituted with 5% glu...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com