A kind of synthetic method of 5-azacytosine
An azacytosine and a synthesis method technology, applied in the field of organic synthesis, can solve the problems of increased difficulty in process safety control and waste liquid treatment, large amount of organic waste and waste water, high environmental pollution pressure, etc., and achieves easy biochemical degradation treatment, The effect of reducing production costs and reducing side reactions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0043] (1) Into a 10L autoclave, 1.5Kg of anhydrous formic acid (99.5%), 2.5Kg of dicyandiamide and p-toluenesulfonic acid catalyst (0.005Kg) were sequentially added. Under the state of sealing and stirring, pressurize the reactor and slowly raise the temperature to 110°C. After the reaction is completed, slowly lower the reaction temperature to room temperature to obtain a white solid.
[0044] (2) Transfer the white solid to a 50L glass reactor, and add 10L of dilute hydrochloric acid with a mass concentration of 5%. Heat at reflux for 30 minutes until the solution becomes clear. Filter the mixed liquid through a filter cartridge to a crystallization kettle. Add 30% ammonia to the filtrate to adjust the pH to 6 and precipitate white crystals. After centrifugation, dry in a hot air oven to obtain 5 -Azacytosine 2.8 kg (yield of 85% based on dicyandiamide).
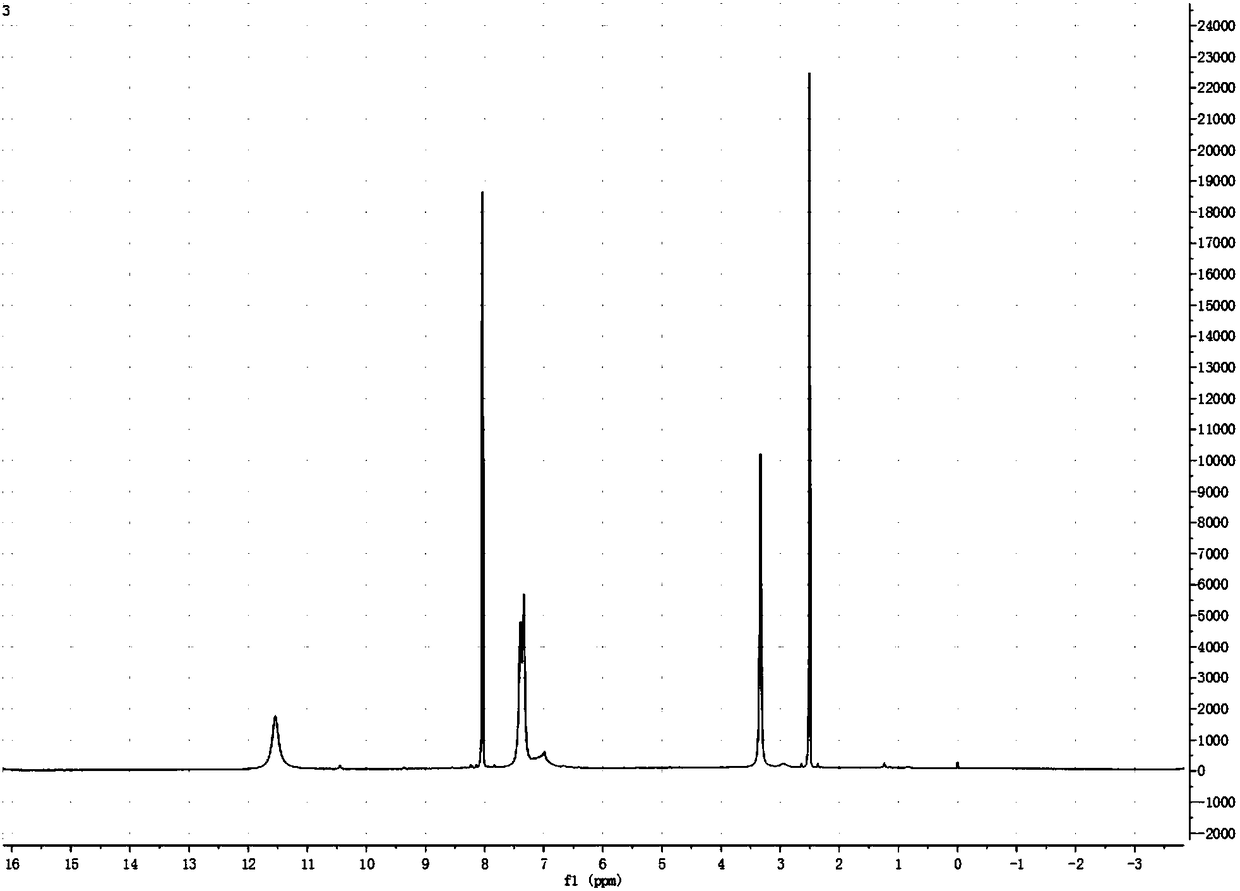
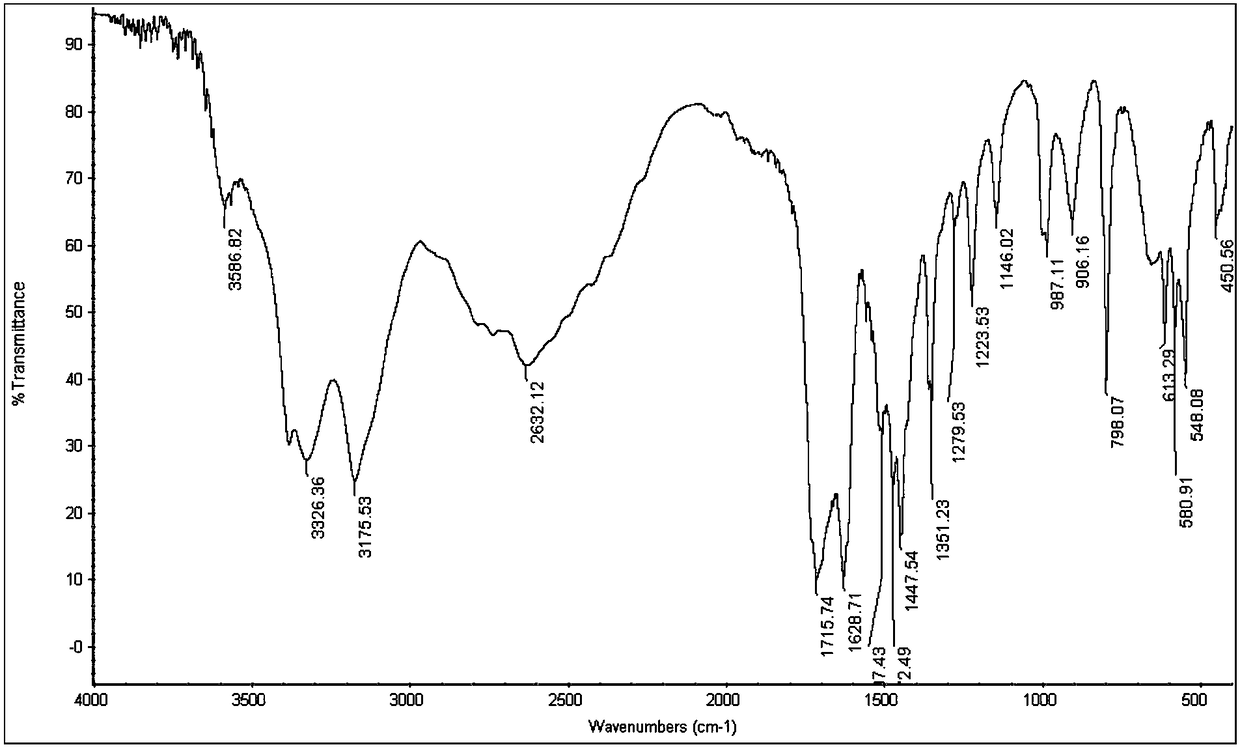
[0045] by figure 1 with figure 2 It can be seen that the product prepared by the present invention is 5-azacytosine, which...
Embodiment 2
[0047] (1) Into a 10L autoclave were sequentially added 1.3Kg of anhydrous formic acid (98%), 2.5Kg of dicyandiamide and p-toluenesulfonic acid catalyst (0.005Kg). Under the state of sealing and stirring, pressurize the reactor and slowly raise the temperature to 100°C. After the reaction is completed, slowly lower the reaction temperature to room temperature to obtain a white solid.
[0048] (2) Transfer the white solid to a 50L glass reactor, and add 10L of dilute hydrochloric acid with a mass concentration of 5%. Heat at reflux for 30 minutes until the solution becomes clear. Filter the mixed liquid through a filter cartridge to a crystallization kettle. Add 30% ammonia to the filtrate to adjust the pH to 6 and precipitate white crystals. After centrifugation, dry in a hot air oven to obtain 5 -2.65 kg of azacytosine (yield of 80% based on dicyandiamide).
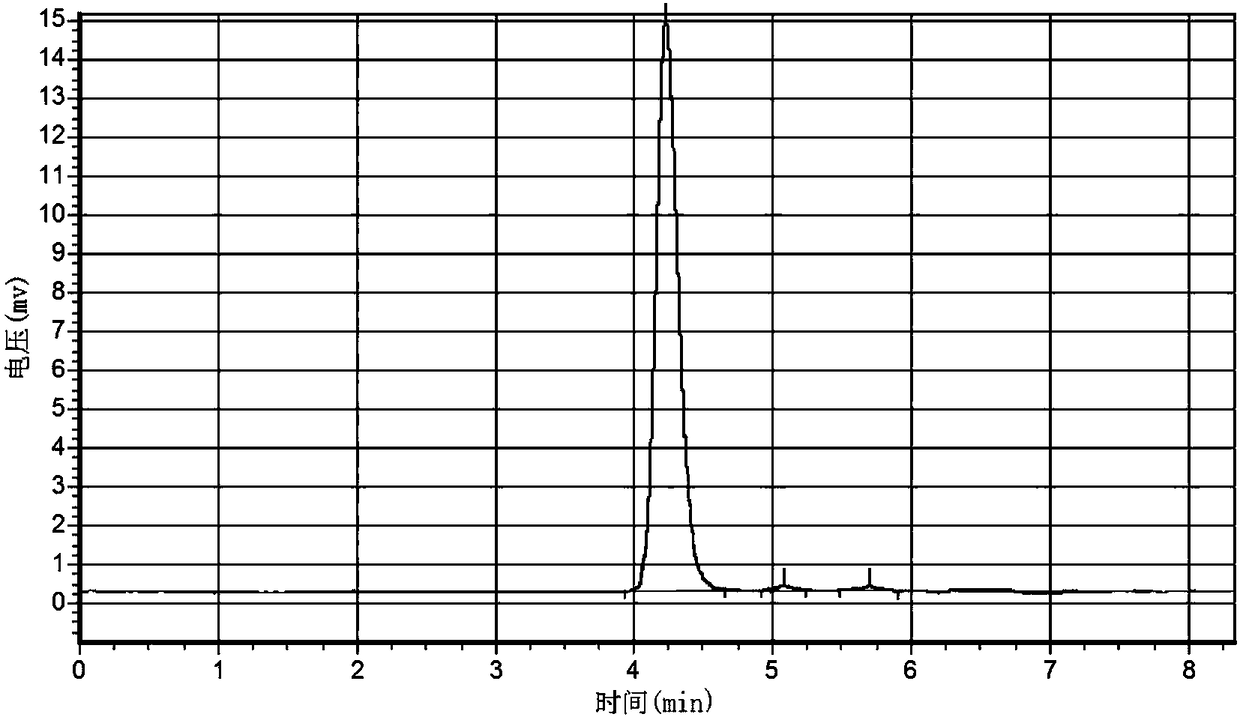
[0049] by Figure 4 It can be seen from the high performance liquid chromatogram that the purity is higher than 95%.
Embodiment 3
[0051] (1) Add anhydrous formic acid (99.5%) 2.0Kg, dicyandiamide 2.5Kg and p-toluenesulfonic acid catalyst (0.005Kg) into a 10L autoclave. Under the state of sealing and stirring, pressurize the reactor and slowly raise the temperature to 120°C. After the reaction is completed, slowly lower the reaction temperature to room temperature to obtain a white solid.
[0052] (2) Transfer the white solid to a 50L glass reactor, and add 10L of dilute hydrochloric acid with a mass concentration of 5%. Heat at reflux for 30 minutes until the solution becomes clear. Filter the mixed liquid through a filter cartridge to a crystallization kettle. Add 30% ammonia to the filtrate to adjust the pH to 6 and precipitate white crystals. After centrifugation, dry in a hot air oven to obtain 5 -Azacytosine 2.8 kg (yield of 85% based on dicyandiamide).
PUM
| Property | Measurement | Unit |
|---|---|---|
| quality score | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com