Preparation method of high-substitution-degree acetylated sodium hyaluronate
A technology of sodium hyaluronate and hyaluronic acid, which is applied in the field of preparation of acetylated hyaluronic acid with high substitution degree, can solve the problems of lack of activation or activation principle in amide reaction, low degree of acetylation, large amount of product loss, etc. The effect of reducing the introduction of impurities, improving the degree of acetylation, and avoiding environmental pollution
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
specific Embodiment approach
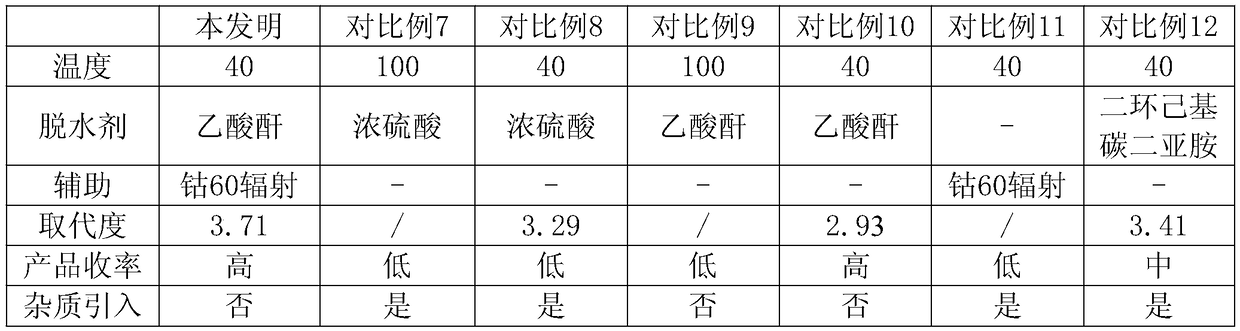
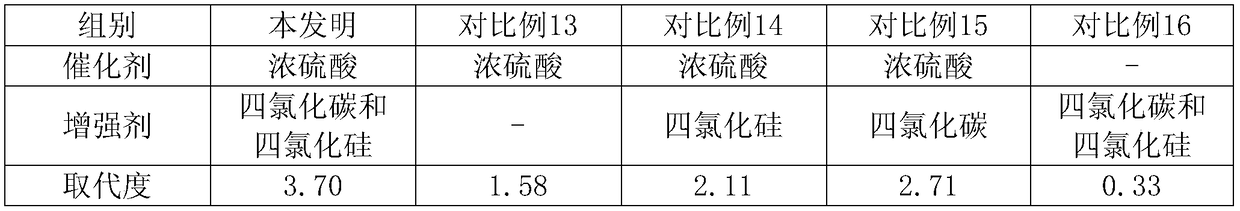
[0027] A specific embodiment of the present invention: the preparation method of the highly substituted acetylated sodium hyaluronate is characterized in that it is prepared from hyaluronic acid with a molecular weight through I activation separation, II dehydration reaction and III acylation reaction;
[0028] Firstly, hyaluronic acid is subjected to I activation and separation operation, specifically: adding hyaluronic acid with a molecular weight of 900,000 to an acetone solution of 8% triethylamine and reacting for 50 minutes, because the product is insoluble in acetone solution, some impurities and reaction The product is easily separated from the product, reducing the introduction of impurities into the reactant. After filtration, the precipitate is taken and washed with acetic acid for 1-2 times, and the solid is separated;
[0029] The set activation process promotes the progress of III acylation reaction in the following process, thereby greatly improving the degree of...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com