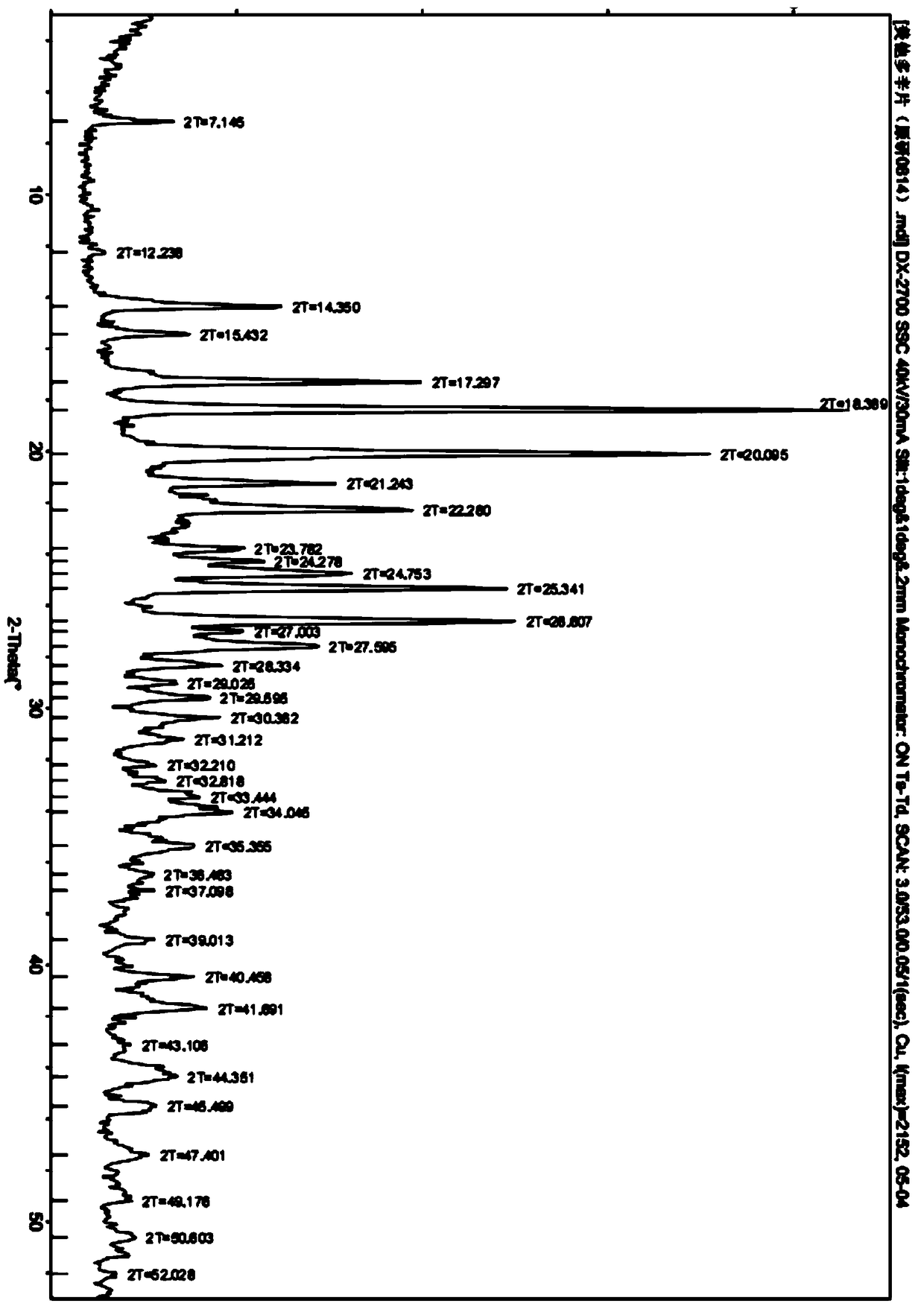
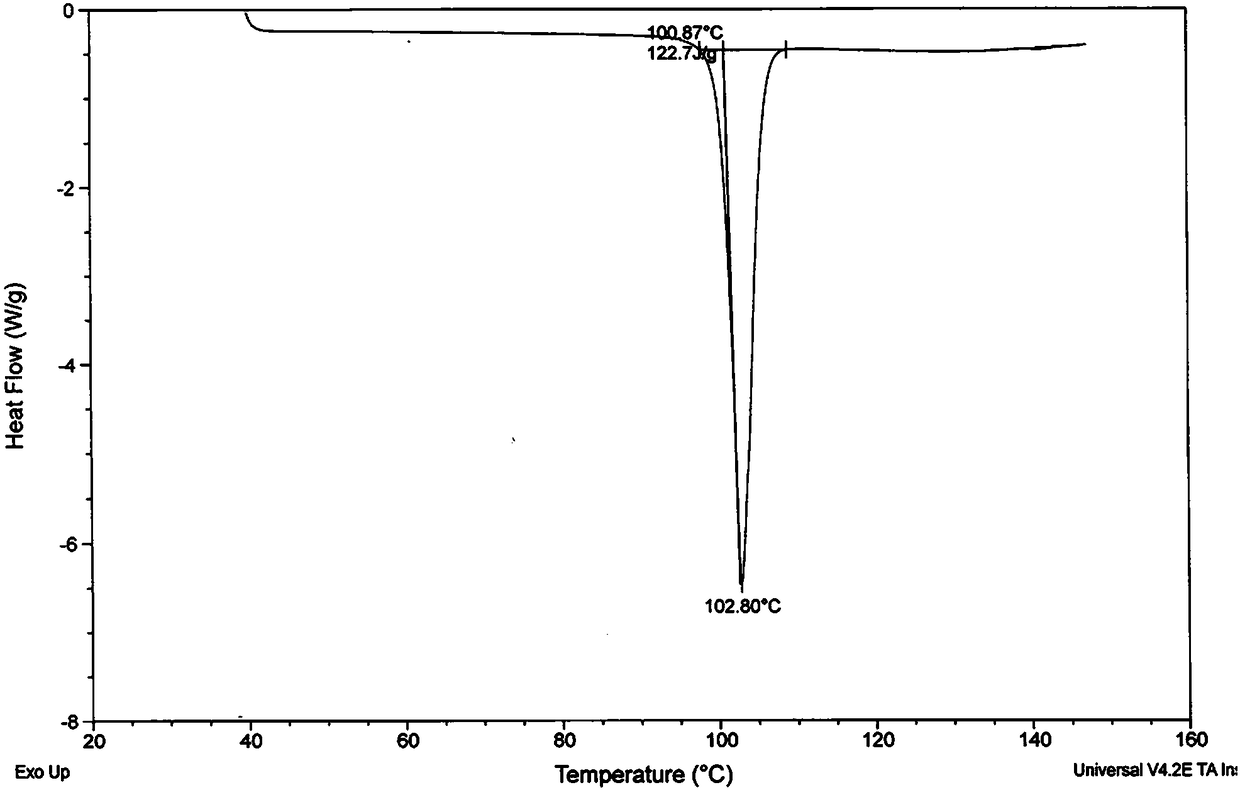
A kind of one-step synthesis method of pharmaceutical grade metadoxine
A synthetic method and advanced beauty technology, applied in the direction of organic chemistry, organic chemistry, etc., can solve the problems of inconvenient operation, high price, difficult quality control, etc., and achieve the effect of easy transportation and storage, low solvent residue and good fluidity.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0059] 1, the preparation of metadoxine of the present invention
[0060] A. Add 30.0Kg vitamin B6 in the 500L reactor, add 90.0Kg isopropanol, and add 29.4Kg of pre-prepared 20% sodium hydroxide solution into the system under stirring (preparation process: 5.88Kg sodium hydroxide is dissolved in 23.52Kg purified water), the temperature of the feed liquid during the addition process was kept below 35°C, and the flow addition was completed in 1 to 2 hours. After the addition was completed, continue to stir for 30 minutes;
[0061] B. centrifuge, collect the mixed mother liquor of isopropanol and water;
[0062] C. Add 18.8Kg of L-pyroglutamic acid to the above mixed mother liquor solution, stir well, and react at 25°C for 30 minutes; cool down to 0-5°C, and keep for 30-60 minutes;
[0063] D. Centrifuge, wash with a small amount of isopropanol, dry the obtained solid at 35°C for 2 hours, then heat up to 45°C and dry for 10 hours to obtain 44.9Kg of white solid, which is a qual...
Embodiment 2
[0079] Embodiment 2 Preparation of metadoxine of the present invention
[0080] A. Add 10.0Kg vitamin B6 in the 100L reactor, add 20.0Kg isopropanol, and add 9.5Kg of 20% sodium hydroxide solution prepared in advance to the system under stirring (preparation process: 1.9Kg sodium hydroxide is dissolved in 7.6Kg purified water), the temperature of the feed liquid during the addition process was kept below 35°C, and the flow addition was completed in 0.5 to 1 hour. After the addition was completed, continue to stir for 30 minutes;
[0081] B. centrifuge, collect the mixed mother liquor of isopropanol and water;
[0082] C. Add 6.29Kg of L-pyroglutamic acid to the above mixed mother solution, stir well, and react at 35°C for 30 minutes; cool down to 0-5°C, and keep for 30-60 minutes;
[0083] D. Centrifuge, wash with a small amount of isopropanol, dry the obtained solid at 35°C for 3 hours, then heat up to 45°C and dry for 7 hours to obtain 14.8Kg of white solid, which can be ob...
Embodiment 3
[0084] Embodiment 3 Preparation of metadoxine of the present invention
[0085] A. Add 1.0Kg vitamin B6 in the 10L three-necked reaction flask, add 1.0Kg isopropanol, and add 0.94Kg of 20% sodium hydroxide solution prepared in advance to the system under stirring (preparation process: 0.188Kg sodium hydroxide dissolved in 0.752Kg purified water), the temperature of the feed liquid during the addition process was kept below 35°C, and the addition was completed within 20 minutes. After the addition was completed, continue to stir for 30 minutes;
[0086] B. centrifuge, collect the mixed mother liquor of isopropanol and water;
[0087] C. Add 0.63Kg of L-pyroglutamic acid to the above mixed mother liquor solution, stir well, and react at 30°C for 30 minutes; cool down to 0-5°C, and keep for 30-60 minutes;
[0088] D. Centrifuge, rinse with a small amount of isopropanol, dry the obtained solid at 35°C for 2 hours, then heat up to 45°C and dry for 6 hours to obtain 1.53Kg of white...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com