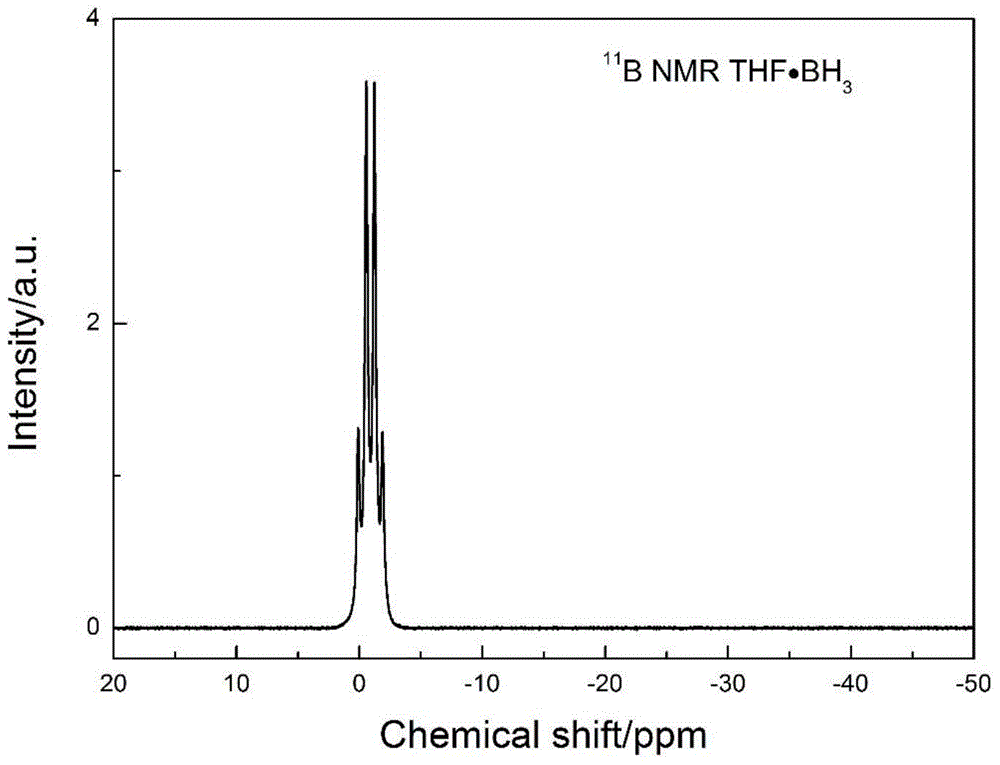
Synthesis method of borane-solvent complex
A synthesis method and borane technology, applied in chemical instruments and methods, metal hydrides, inorganic chemistry, etc., can solve the problems of high risk, high toxicity, explosion, etc., and achieve convenient operation, low cost and low toxicity. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0027] The operation steps are described as follows:
[0028] 1) Weigh 11.4237g of sodium borohydride and dissolve it in 300ml of tetrahydrofuran.
[0029] 2) Weigh 41.7182 g of lead chloride, and add it into a tetrahydrofuran solution of sodium borohydride.
[0030] 3) Stir the solution at room temperature.
[0031] 4) Until the sodium borohydride is completely consumed.
[0032] The reaction equation is: 2NaBH 4 +2THF+PbCl 2 =2NaCl+Pb+2THF·BH 3 +H 2
[0033] 5) Rotary evaporation, in the way of collecting in the cold trap, the borane tetrahydrofuran complex is separated from the precipitate.
[0034] 6) The solution collected in the cold trap is the borane tetrahydrofuran complex.
Embodiment 2
[0036] The operation steps are described as follows:
[0037] 1) Weigh 16.2333g of potassium borohydride and dissolve it in 300ml of tetrahydrofuran.
[0038] 2) Weigh 26.6975 g of bismuth trifluoride, and add it into a tetrahydrofuran solution of potassium borohydride.
[0039] 3) Stir the solution at room temperature.
[0040] 4) Until potassium borohydride is completely consumed.
[0041] The reaction equation is: 6KBH 4 +6THF+2BiF 3 =6KF+2Bi+6THF·BH 3 +3H 2
[0042] 5) Rotary evaporation, in the way of collecting in the cold trap, the borane tetrahydrofuran complex is separated from the precipitate.
[0043] 6) The solution collected in the cold trap is the borane tetrahydrofuran complex.
Embodiment 3
[0045] 1) Weigh 11.4812g of sodium borohydride and dissolve it in 300ml of tetrahydrofuran.
[0046] 2) Weigh 29.7753 g of cuprous chloride, and add it to a tetrahydrofuran solution of sodium borohydride.
[0047] 3) Stir the solution at room temperature.
[0048] 4) Until the sodium borohydride is completely consumed.
[0049] The reaction equation is: 2NaBH 4 +2THF+2CuCl=2NaCl+2Cu+2THF·BH 3 +H 2
[0050] 5) Centrifuge the solution at high speed.
[0051] 6) The collected supernatant is the borane tetrahydrofuran complex.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com