Method for correcting excessive adsorption quantity of adsorbate gas in isothermal adsorption experiment
A technology of excess adsorption and isothermal adsorption, applied in the field of shale gas exploration, to achieve objective evaluation results
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
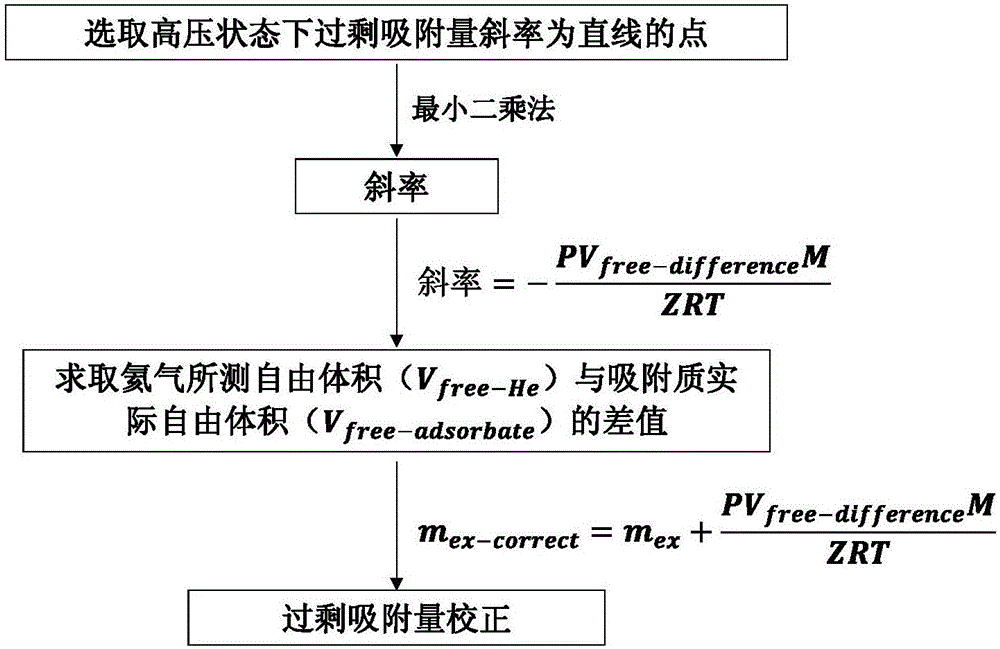
[0029] The schematic diagram of volumetric isothermal adsorption experiment equipment is as follows: Figure 4 As shown, the volume of the reference cell is known, and the free volume in the sample cell (V free-He ), and the actual free volume corresponding to the adsorbate gas molecules (V frfree-adsorbate ) less than V free-He , the difference between the two is recorded as V free-difference ,but
[0030] V free-He =V free-adsorbate +V free-difference (a)
[0031] In the experiment, the total gas volume injected into the sample cell n total It can be obtained from the change of the pressure in the reference cell, and the excess adsorption amount can be obtained by making a difference with the amount of gas existing in the bulk phase density in the free volume:
[0032] n e x = n t o t a l ...
Embodiment 2
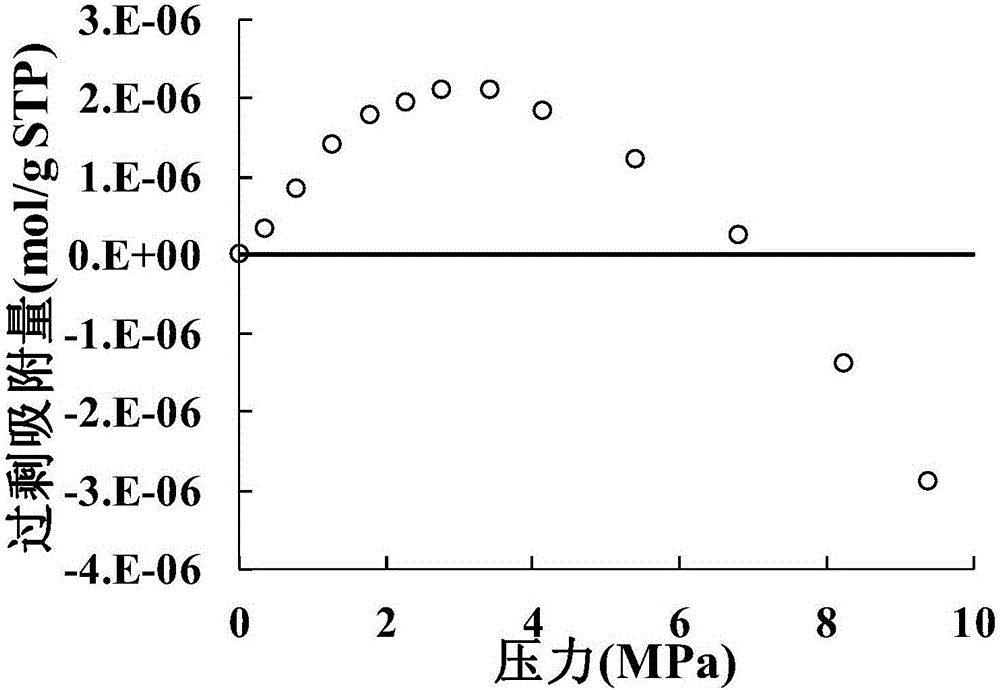
[0040] Due to the inability to control V during the experiment free-adsorbate Accurate measurements were made, so the accuracy of the above scheme cannot be verified. While the V in the molecular simulation process free-adsorbate Can get, also can get the exact value of excess adsorption. In order to verify the accuracy of the method of the present invention, the V in the simulation processfree-adsorbate It is artificially increased to simulate the high free volume measured by helium during the experiment. Use artificially increased V free-adsorbate represents the V during the experiment free-He . Due to the artificial adjustment of the free volume, the excess adsorption amount is lower than the real value, and the excess adsorption amount is less than 0 when the pressure exceeds 30 MPa (such as Figure 5 shown).
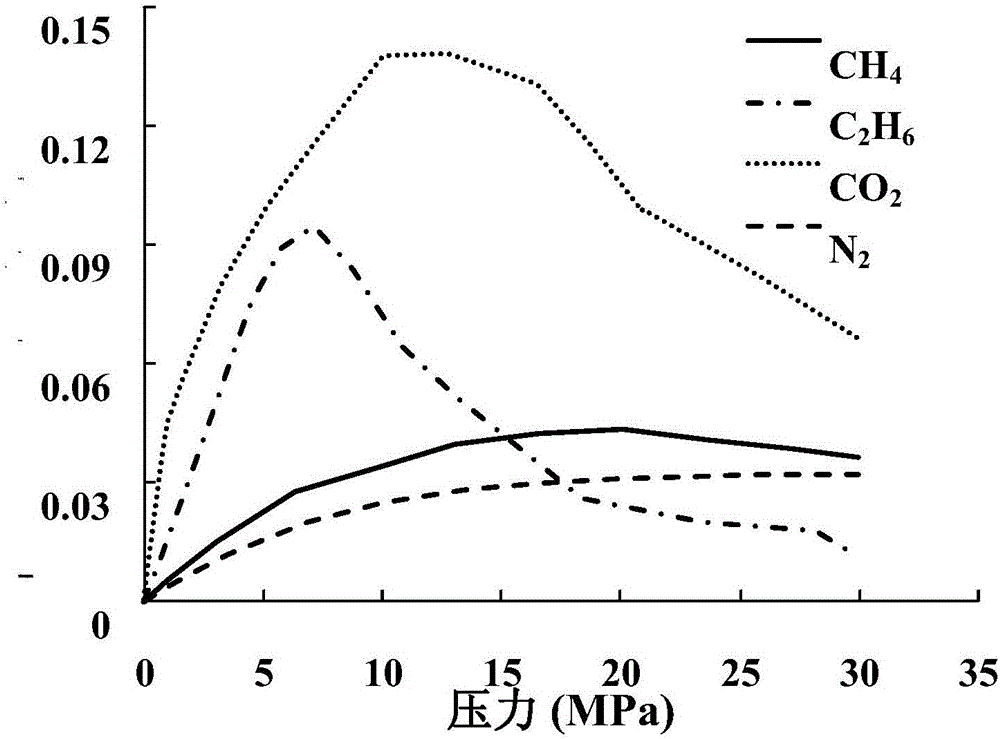
[0041] C below 2 h 6 Taking the adsorption curve on illite as an example, the problem of low excess adsorption caused by the free volume being higher than th...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com