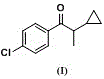
Synthetic method of cyproconazole intermediate
A technology of cyproconazole and its synthesis method, which is applied in the field of chemistry and chemical engineering, can solve problems such as complex process, high economic cost, and high risk, and achieve the effects of high process fluency, high total reaction yield, and strong operability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0028] The synthesis of embodiment 1 compound (III)
[0029]
[0030] In a 500 ml single-necked bottle, add 56g (0.4mol) of p-chlorobenzaldehyde, 150g of toluene, 63.84g (0.84mol) of ethylene glycol methyl ether, and 1.72g (0.01mol) of p-toluenesulfonic acid, and reflux to separate water After 6 hours, triethylamine was added to adjust the pH=7-8, and the solvent was distilled off to obtain 108.2 g of compound (III), with a purity of 97.4% and a yield of 96.3%.
Embodiment 2
[0031] The synthesis of embodiment 2 compound (III)
[0032] In a 500 ml single-necked bottle, add 56g (0.2mol) of p-chlorobenzaldehyde, add 170g of benzene, add 63.84g (0.84mol) of ethylene glycol methyl ether, add 18.25g (0.01mol) of phosphomolybdic acid, reflux and divide water for 6 After 1 hour, triethylamine was added to adjust the pH=7-8, and the solvent was evaporated to obtain 104.9 g of compound (III), with a purity of 96.8% and a yield of 92.6%.
Embodiment 3
[0033] The synthesis of embodiment 3 compound (III)
[0034] In a 500 ml single-necked bottle, add 56g (0.2mol) of p-chlorobenzaldehyde, 180g of toluene, 63.84g (0.84mol) of ethylene glycol methyl ether, and 1.90g (0.01mol) of titanium tetrachloride, and reflux to separate water After 6 hours, triethylamine was added to adjust the pH to 7-8, and the solvent was evaporated to obtain 103.7 g of compound (III), with a purity of 96.4% and a yield of 91.2%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com