A kind of ring metal iridium complex and its preparation method and its application as protein staining agent
A technology of iridium complexes and ring metals, which is applied in the field of biological analysis, can solve problems such as complex rinsing steps, low dyeing sensitivity, and poor sensitivity of Coomassie Brilliant Blue, and achieve high dyeing sensitivity, simple preparation method, and good dyeing effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0051] Example 1 Synthesis of ionic luminescent ring metal iridium complexes
[0052]
[0053] First, after reacting iridium trichloride trihydrate crystalline compound with C^N bidentate ligand (compound 1) in ethylene glycol ether solvent for 24 hours, a transition metal chloride bridge compound (compound 2) was obtained, and then the chlorine bridge The compound was reacted with N^N bidentate ligand compound (compound 3), the solvent was spin-dried, and the final ionic luminescent ring metal iridium compound (compound 4) was obtained by column chromatography. Yield: 68%. Mass spectrum: experimental value [M-Cl] + 655.1610, theoretical value [M-Cl] + 655.1601.
[0054] By replacing the C^N bidentate ligand and N^N bidentate ligand compounds, ion-type luminescent ring metal iridium complexes with multiple structures can be obtained.
[0055] It is also possible to combine the chloride ion iridium complex obtained above with NaBr, KI or KPF 6 The reaction yields cyclom...
Embodiment 2
[0056] Example 2 Preparation of electrically neutral and luminescent ring metal iridium complexes
[0057]
[0058] Iridium acetylacetonate Ir(acac) 3 Heated and reacted with C^N bidentate ligand compound 1 for 24 hours, and separated by column chromatography to obtain electrically neutral luminescent ring metal iridium complex 2. The yield was 60%. Mass Spectrum: Experimental [M+H] + 655.1510, theoretical value [M+H] + 655.1599.
[0059] By replacing the C^N bidentate ligand compound, multiple structures of electrically neutral luminescent ring metal iridium complexes can be obtained.
Embodiment 3
[0061] The structural formula of the protein stain used is:
[0062]
[0063] The fluorescence quantum efficiency of this compound is 37%.
[0064] The ring metal iridium complex of the present invention is used for protein staining and gel test, the process is as follows:
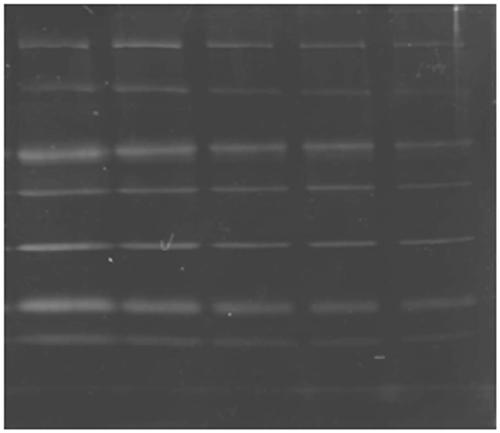
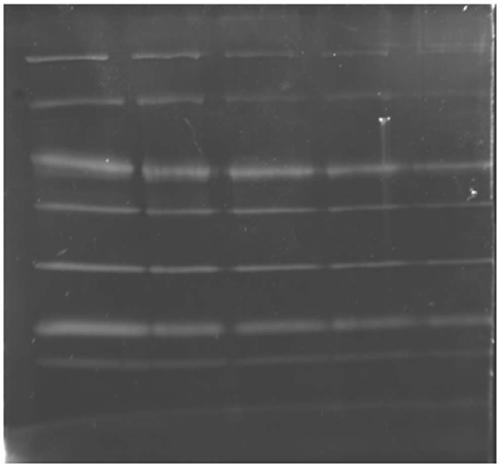
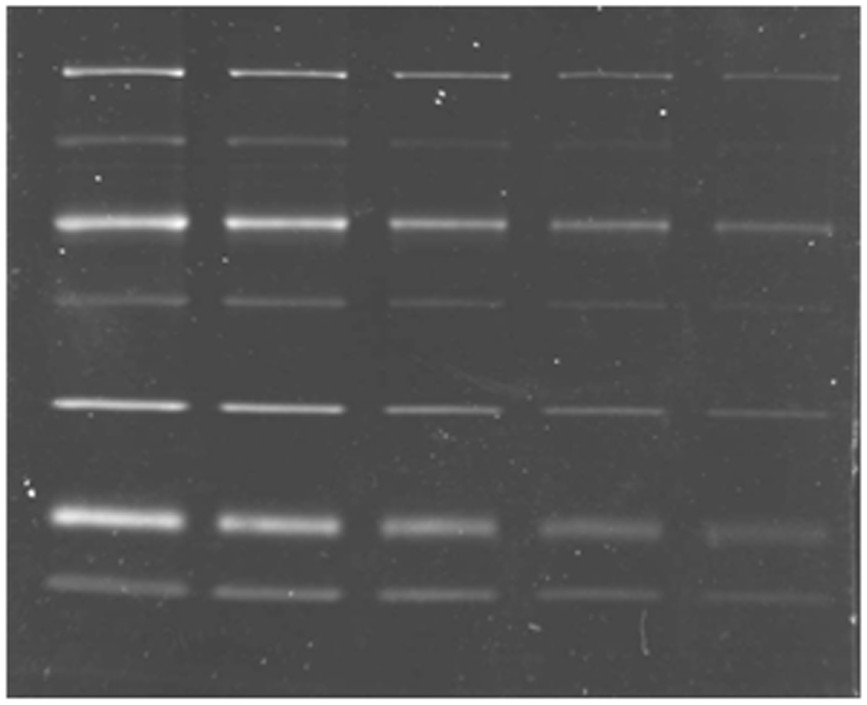
[0065] 1. After the protein sample was subjected to SDS-PAGE, it was fixed in 30% ethanol and 10% acetic acid;
[0066] 2. Rinse the glue in 20% ethanol for 3 times, 30 minutes each time;
[0067] 3. Dilute the protein dye luminescent iridium complex from the mother liquor (with DMSO as the solvent) to 1 micromol with pure water, and then place the gel in it for staining for 6 hours;
[0068] 4. Take out the gel after the third step and place it in the gel imaging system for imaging. Choose 312nm as excitation wavelength and 515nm as emission wavelength.
[0069] Stain commercially available protein markers (molecular weight: 116KDa, 66.2 KDa, 45 KDa, 35 KDa, 25 KDa, 18.4 KDa, 14.4 KDa) separated by...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com