Iridium-selenium polypyridine complex and its preparation method and application
A technology of polypyridine and complexes, applied in the field of living cell staining, to achieve the effect of enhancing cell transmembrane ability, strong cell penetration ability, and short incubation time
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
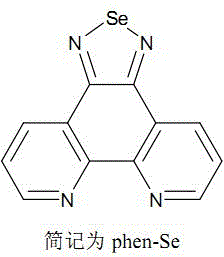
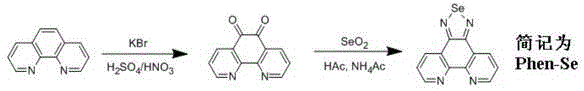
[0029] Embodiment 1 Preparation of ligand selenium-5,6-diamine-1,10-phenanthroline (phen-Se)
[0030] The synthesis of ligand phen-Se can be prepared by referring to existing literature (Qian Li, Dongdong Sun, Yanhui Zhou, Du Liu, Qianling Zhang and Jie Liu, Inorg.Chem.Commun., 2012, 20, 142), and its synthetic route was carried out Partial optimization and adjustment reduces the synthesis steps, making the synthesis simpler and faster with high yield. Only two steps are required, and the first step is a common and mature reaction for synthesizing polypyridine ligands.
[0031] (1) 1,10-phenanthroline-5,6-dione
[0032] It can be prepared by referring to the literature (M.Yamada, Y.Tanaka, Y.Yoshimato, S.Kuroda and I.Shimao, Bull.Chem.Soc.Jpn., 1992, 65, 1006). Under cooling in an ice bath, drop 40 cm 3 Concentrated sulfuric acid and 20cm 3 Mixed solution of concentrated nitric acid while magnetically stirring. After the dropwise addition was completed, the ice bath was r...
Embodiment 2
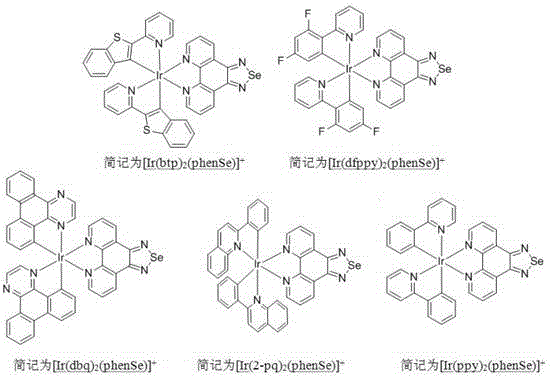
[0035] Example 2 Complex [Ir(ppy) 2 (phenSe)] + (PF 6 ) - , [Ir(dfppy) 2 (phenSe)] + (PF 6 ) - , [Ir(btp) 2 (phenSe)] + (PF 6 ) - , [Ir(2-pq) 2 (phenSe)] + (PF 6 ) - ,[Ir(dbq) 2 (phenSe)] + (PF 6 ) - Synthetic method:
[0036] (1)[(dfppy) 2 Ir(μ-Cl)] 2 Synthesis
[0037] The synthesis of this compound can be prepared by referring to existing literature (T.Peng, Y.Yang, Y.Liu, D.Ma, Z.Hou and Y.Wang, Chem.Commun., 2011, 47, 3150). Weigh IrCl 3 0.33g (about 1.1mmol), 2-(2,4-difluorophenyl)pyridine (dfppy) 0.501g (2.62mmol), mixed in 40mL ethylene glycol ether and water (V1:V2=3:1) liquid, reflux at 120°C for about 24 hours under the protection of argon. After cooling to room temperature, an appropriate amount of water was added, and the solid precipitate was collected by suction filtration, washed with n-hexane and ether several times, and dried to obtain a yellow-green powder with a yield of 53.5%.
[0038] (2)[(ppy) 2 Ir(μ-Cl)] 2 Synthesis
[0039] ...
Embodiment 3
[0059] The laser confocal experiment of embodiment 3 iridium complexes
[0060] Cell culture: Hela cells were cultured in DMEM medium containing 10% fetal bovine serum, cells (5'10 8 / L) inoculated in a special glass bottom culture dish for confocal microscope, the diameter of the culture dish is 35mm, the thickness of the cover glass is 0.085~0.13mm, the diameter of the micropore in the center of the dish is 10mm, 5% CO 2 and 95% air condition, cultured at 37°C, and grown adherently for 24 hours.
[0061] Confocal Microscopy-Cell Imaging: Hela cells with complexes {5 μM, [Ir(ppy)2(phenSe)] + (PF 6 - )(A), [Ir(dfppy)2(phenSe)] + (PF 6 - )(B), [Ir(btp)2(phenSe)] + (PF 6 - )(C), [Ir(2-pq)2(phenSe)] + (PF 6 - )(D), [Ir(dbq)2(phenSe)] + (PF 6 - )(E)} incubate for 30min, and then incubate with mitochondrial green fluorescent dye Mitotracker-Green (50nM) for a certain period of time, suck out the culture medium, and then wash with PBS buffer for 3 to 4 times. For ima...
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
| diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com