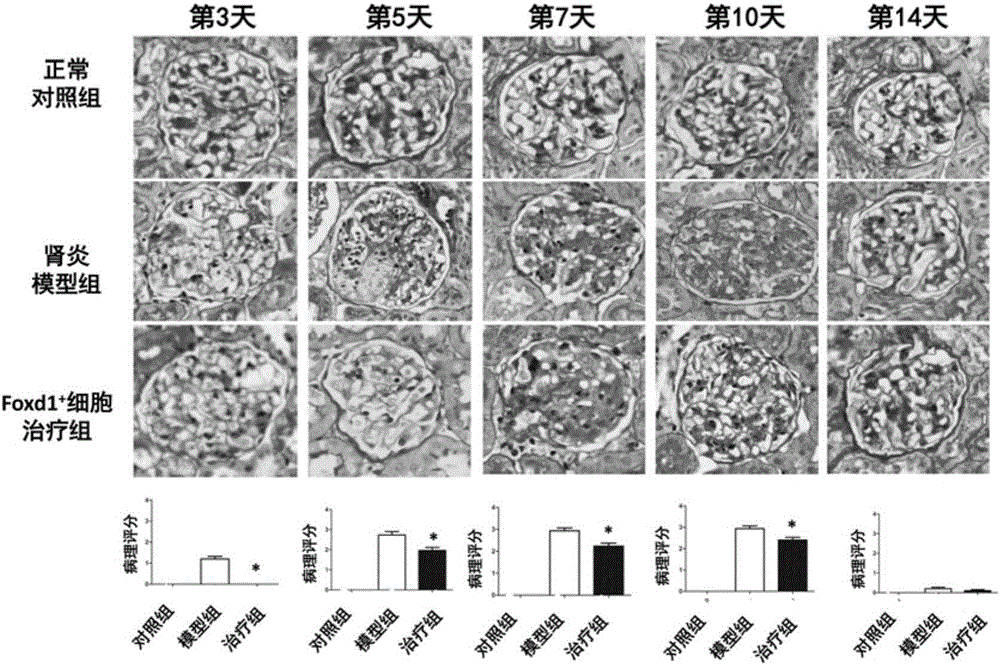
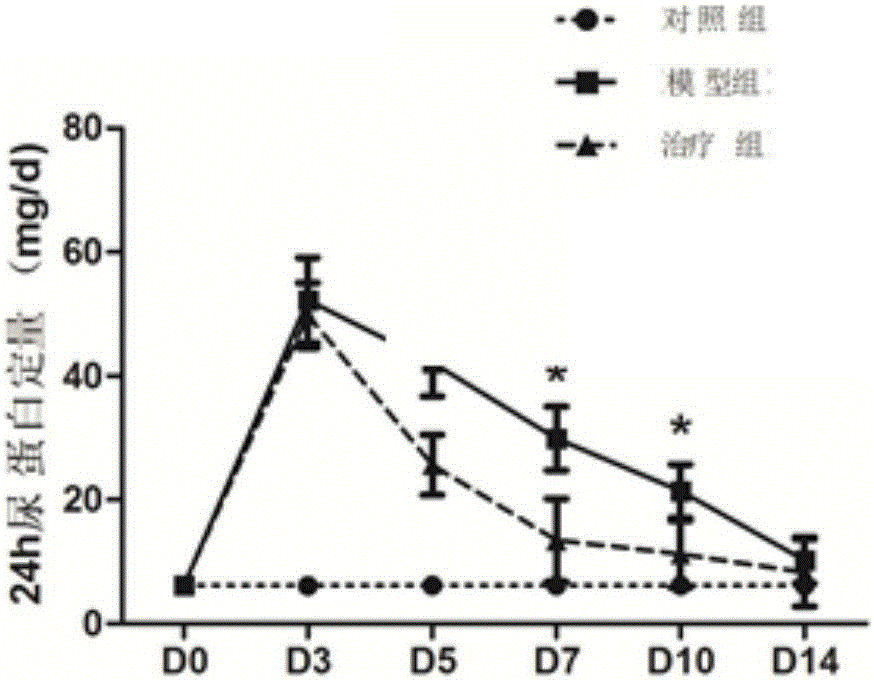
Medicine for treating mesangial proliferative glomerulonephritis
A technology for treating glomerulonephritis and drugs, which is applied in the field of stem cell therapy, and can solve the problems of large toxic and side effects of cytotoxic drugs, limiting the application of hormones and cytotoxic drugs, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0021] Example 1Foxd1 + Effect of metanephric mesenchymal cells on mesangial proliferative glomerulonephritis
[0022] 1. Foxd1 + Preparation of metanephric mesenchymal cells
[0023] 1.1 Subculture
[0024] 1) Use Foxd1 cre Transgenic mice with DTR flox Transgenic mice were mated to screen Foxd1-cre; DTR-flox double transgenic embryonic mice at E13.5 days, and metanephric mesenchymal cells were extracted and inoculated in stem cell culture medium at 37°C, 5% CO 2 After culturing for 60-72 hours, add diphtheria toxin with a final concentration of 100-150ng / ml to the culture medium, and change the medium with stem cell culture medium containing 100-150ng / ml diphtheria toxin every 48 hours until cultured 5-7 days, when the cells are 80% confluent, passage at a ratio of 1:2;
[0025] 2) Digest the cells with 0.25% trypsin (2.5ml / 25cm 2 Culture bottle, 5ml / 75cm 2 culture flask), incubate at 37°C for 3 minutes, and stop digestion with 2 times the volume of trypsin containin...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com